
Environment Protection and Biodiversity Conservation (National Recovery Plan for the Wollemi Pine (Wollemia nobilis)) Instrument 2025
I, Tanya Plibersek, the Minister for the Environment and Water, make the National Recovery Plan for the Wollemi Pine (Wollemia nobilis) in the following instrument, jointly with New South Wales.
Dated 18/03/2025
Tanya Plibersek
Minister for the Environment and Water
- Name
This instrument is the Environment Protection and Biodiversity Conservation (National Recovery Plan for the Wollemi Pine (Wollemia nobilis)) Instrument 2025.
- Commencement
This instrument commences on the day after it is registered.
- Authority
This instrument is made under subsection 269A(3) of the Environment Protection and Biodiversity Conservation Act 1999.
- Jointly made recovery plan
The National Recovery Plan for the Wollemi Pine (Wollemia nobilis) in this instrument is jointly made with New South Wales, as agreed by the Minister for the Environment (New South Wales).

© Commonwealth of Australia 2025
Ownership of intellectual property rights
Unless otherwise noted, copyright (and any other intellectual property rights) in this publication is owned by the Commonwealth of Australia (referred to as the Commonwealth).
Creative Commons licence
All material in this publication is licensed under a Creative Commons Attribution 4.0 International Licence except content supplied by third parties, logos and the Commonwealth Coat of Arms.
Inquiries about the licence and any use of this document should be emailed to copyright@dcceew.gov.au.

Cataloguing data
This publication (and any material sourced from it) should be attributed as: Wollemi Pine Recovery Team 2025, National Recovery Plan for the Wollemi Pine (Wollemia nobilis), Department of Climate Change, Energy, the Environment and Water, Canberra, CC BY 4.0.
This publication is available at dcceew.gov.au/publications.
Department of Climate Change, Energy, the Environment and Water
GPO Box 3090 Canberra ACT 2601
Telephone 1800 920 528
Web dcceew.gov.au
Disclaimer
The Australian Government acting through the Department of Climate Change, Energy, the Environment and Water has exercised due care and skill in preparing and compiling the information and data in this publication. Notwithstanding, the Department of Climate Change, Energy, the Environment and Water, its employees and advisers disclaim all liability, including liability for negligence and for any loss, damage, injury, expense or cost incurred by any person as a result of accessing, using or relying on any of the information or data in this publication to the maximum extent permitted by law.
Acknowledgements
The Department of Climate Change, Energy, the Environment and Water acknowledges that the preparation of this recovery plan was made possible by the contributions of numerous individuals and organisations, including the Wollemi Pine Recovery Team, NSW National Parks and Wildlife Service and NSW Department of Climate Change, Energy, the Environment and Water. We thank those who have contributed for their support.
Acknowledgement of Country
Our department recognises the First Peoples of this nation and their ongoing connection to culture and Country. We acknowledge Aboriginal and Torres Strait Islander Peoples as the Traditional Owners, Custodians and Lore Keepers of the world's oldest living culture and pay respects to their Elders past, and present.
Image credits
Cover Page: Adult trees (© Copyright, Jaime Plaza, Royal Botanic Gardens and Domain Trust)
Page viii: Adult foliage with branch scars (© Copyright, Jaime Plaza, Royal Botanic Gardens and Domain Trust)
Page 9: Adult trees (© Copyright, Phil Lamrock, NSW National Parks and Wildlife Service)
Page 18: ABGMA living collection (© Copyright, Maureen Phelan, Royal Botanic Gardens and Domain Trust)
Page 25: Post-fire resprouting (© Copyright, Berin Mackenzie, NSW DCCEEW)
Page 29: Seedling (© Copyright, Berin Mackenzie, NSW DCCEEW)
Page 33: Juvenile foliage (© Copyright, Jaime Plaza, Royal Botanic Gardens and Domain Trust)
Page 39: Seedling (© Copyright, Jaime Plaza, Royal Botanic Gardens and Domain Trust)
Page 45: Adult tree with female cones (© Copyright, Jaime Plaza, Royal Botanic Gardens and Domain Trust)
Contents
Executive summary..........................................................iv
Acronyms.................................................................vi
Acknowledgements..........................................................vii
1 General information......................................................1
1.1 Introduction........................................................1
1.2 Historical context.....................................................1
1.3 Legislative, policy and planning context......................................2
1.4 Evaluation of previous recovery plan........................................7
1.5 Stakeholders........................................................7
1.6 First Nations connections................................................8
1.7 Benefits and impacts...................................................8
2 Species information......................................................10
2.1 Taxonomy.........................................................10
2.2 Species Description...................................................11
2.3 Distribution........................................................13
2.4 Habitat...........................................................19
2.5 Biology and ecology..................................................20
2.6 Threats...........................................................26
3 Recovery planning.......................................................30
3.1 Vision............................................................30
3.2 Recovery objectives, performance criteria and actions............................30
3.3 Implementation, costs and monitoring......................................41
3.4 Evaluation of the recovery plan...........................................44
References................................................................46
Appendix 1: Summary of research into the Wollemi Pine.................................54
Appendix 2: Evaluation of progress towards objectives from previous recovery plan..............56
Tables
Table 1: Wollemia nobilis post-fire population structure..................................15
Table 2: Actions to protect and maintain the wild population and its habitat....................31
Table 3: Actions to improve understanding of Wollemi Pine biology and ecology..................34
Table 4: Actions to establish and maintain ex situ collections...............................36
Table 5: Actions to establish and maintain translocated stands.............................38
Table 6: Actions to improve community and Indigenous engagement with recovery efforts...........40
Table 7: Indicative priorities, timings and costs ($k) for the implementation of recovery actions........42
FIGURES
Figure 1: Self-coppicing habit of Wollemi Pine.........................................11
Figure 2: Male cone of Wollemi Pine...............................................12
Figure 3: Female cone of Wollemi Pine.............................................13
Figure 4: Location of Wollemi National Park, New South Wales, Australia.......................14
Figure 5: Population size of translocated stands.......................................17
This ‘National Recovery Plan for the Wollemi Pine (Wollemia nobilis)’ presents conservation and management actions necessary to halt decline and support recovery of the Wollemi Pine in its only known natural occurrence.
The Wollemi Pine is listed as Critically Endangered under both the NSW Biodiversity Conservation Act 2016 (BC Act) and the Commonwealth Environment Protection and Biodiversity Conservation Act 1999 (EPBC Act). This plan has been prepared in partnership with the Wollemi Pine Recovery Team, which consists of land managers, researchers and specialists in the biology, ecology, and management of the species. This plan builds on the previous 2006 recovery plan for the species and is designed to align with the Saving Our Species strategy for the Wollemi Pine under the NSW Government’s Saving Our Species (SoS) program.
Population structure and threats
The Wollemi Pine is a long-lived, slow-growing rainforest tree belonging to the 200-million-year-old Araucariaceae family. As of June 2024, it is known from a single wild population in Wollemi National Park that consists of four discrete stands, totalling 45 adult plants and 46 juveniles.
The Wollemi Pine is threatened by demographic and environmental stochasticity due to its extremely small population size and geographic distribution; fire regimes that cause declines in biodiversity (particularly high severity fire and high frequency fire); dieback from the pathogens Phytophthora cinnamomi and P. multivora; unauthorised (illegal) visitation to the wild population resulting in damage to the species and its habitat through trampling of seedlings and juveniles, soil compaction and erosion, introduction of weeds and/or novel pathogens, and spread of existing pathogens; and anthropogenic climate change, including changes to rainfall, temperature, and disturbance regimes such as fires, floods, heatwaves and storms.
The wild Wollemi Pine population and a further three translocated stands of Wollemi Pine were impacted by the 2019–20 bushfires. These fires were mainly active in the understorey, resulting in severe impacts on, and extensive mortality of, seedlings and juveniles. Several adult trees in the wild population also suffered significant damage. Population recovery is being closely monitored and research into tolerable fire regimes for the Wollemi Pine is ongoing. The influence of anthropogenic climate change on the severity and scale of these bushfires together with known sensitivities of Wollemi Pine to other phenomena worsening under climate change (such as heatwaves and droughts), highlights the need to develop greater understanding of the nature of climate change impacts on the wild Wollemi Pine population. Additionally, following the 2019–20 bushfires, feral pig and deer populations increased significantly in the wild Wollemi Pine population’s catchment area. Disturbance by feral animals and the associated dispersal of weed propagules and soil pathogens are seen as emerging threats.
Recovery objectives
In support of the long-term conservation and recovery of the Wollemi Pine, the objectives of this plan are that by 2034:
the wild Wollemi Pine population and its habitat are protected from threatening processes to the extent practicable, and have not decreased in size or quality
understanding of the biology of the Wollemi Pine and co-occurring species in its natural habitat has been improved and informs the conservation of the species and its habitat
representative ex situ collections are established and maintained
translocated stands of Wollemi Pine are maintained, and additional translocations are undertaken, as required
community and Indigenous awareness of the Wollemi Pine and engagement with recovery efforts is maintained and improved.
The implementation of this recovery plan will be monitored and reported on by the members of the Wollemi Pine Recovery Team.
Term | Definition |
ABGMA | Australian Botanic Garden, Mount Annan |
AIS | Asset of Intergenerational Significance |
AOBV | Area of Outstanding Biodiversity Value |
BC Act | NSW Biodiversity Conservation Act 2016 |
BCD | Biodiversity and Conservation Division (NSW DCCEEW) |
BGCI | Botanic Gardens Conservation International |
BGOS | Botanic Gardens of Sydney |
BMBGMT | Blue Mountains Botanic Garden, Mount Tomah |
CAP | Conservation Action Plan |
CSIRO | Commonwealth Scientific and Industrial Research Organisation |
Cwlth DCCEEW | Commonwealth Department of Climate Change, Energy, the Environment and Water |
DBH | Diameter at breast height |
EPBC Act | Commonwealth Environment Protection and Biodiversity Conservation Act 1999 |
GBMWHA | Greater Blue Mountains World Heritage Area |
IUCN | International Union for the Conservation of Nature |
KTP | Key Threatening Process |
NGS | National Geographic Society |
NPW Act | NSW National Parks and Wildlife Act 1974 |
NPWS | NSW National Parks and Wildlife Service |
NSW DCCEEW | NSW Department of Climate Change, Energy, the Environment and Water |
RBGDT | Royal Botanic Gardens and Domain Trust |
SID | Science and Insights Division (NSW DCCEEW) |
SoS | NSW Saving our Species program |
TAP | Threat Abatement Plan |
TSC Act | NSW Threatened Species Conservation Act 1995 |
UNSW | University of New South Wales |
WNP | Wollemi National Park |
The research into, and management of, Wollemi Pine over the past 30 years has been a joint effort of the NSW Department of Climate Change, Energy, the Environment and Water’s (NSW DCCEEW) Science and Insights Division (SID), the NSW National Parks and Wildlife Service (NPWS) and the Royal Botanic Gardens and Domain Trust (RBGDT). This recovery plan is a revision of the 2006 plan and has been the combined effort of many people who have contributed to survey, research, on-ground management and ex-situ conservation of the species. In particular, the Wollemi Pine Recovery Team (currently Tony Auld (UNSW), Steve Clarke, David Coote (BCD), David Crust (NPWS), Berin Mackenzie (SEID), Lisa Menke (NPWS), Cathy Offord (RBGDT), Maureen Phelan (RBGDT), Brett Summerell (RBGDT) and Heidi Zimmer (CSIRO)) would like to thank the following people and organisations:
David Noble (NPWS) and his companions Tony Zimmerman and Michael Casteleyn who discovered the species, and Wyn Jones (NPWS), Ken Hill (RBGDT) and Jan Allen (BMBGMT), who formally described Wollemia nobilis and brought it to the attention of the world.
Sharon Nash and Julie Ravallion (NPWS) who prepared the original 1998 recovery plan and Patricia Meagher (RBGDT) who prepared the revised 2006 recovery plan.
Jan Allen (RBGDT); Wyn Jones, Chris Pavich and Michael Sharp of NPWS; and Hayden Washington, for their early contributions to fieldwork, research, monitoring and recovery planning.
NPWS rangers and field staff, for operation and field support.
NPWS Park Air pilots and crew, for aerial support.
Evan Curtis, Andrew Denham, John Porter, Jane Williamson, Katy Wilkins, Liz Tasker and Mark Tozer (SID); Terry Mazzer and Jessica Peterie (BCD); Ian Allan, Stuart Allan, Stefanie Carusi, Matt Coyne, Adam Pigott, Antony Rivers, Randy Sing, Jessica Wait and Dean Whitton (RBGDT); Stephen Bell and Phil Lamrock for field contributions to the translocation program.
John Benson, Jason Bragg, Peter Cuneo, Graeme Errington, Glen Fensom, Edward Liew, Robert Makinson, Hannah McPherson, Patricia Meagher, Carolyn Porter, Jaime Plaza, Amanda Rollason, Maurizio Rossetto, Joanne Tyler, Veronica Viler and Samantha Yap of RBGDT, for their past and present work in the field or on the propagation and cultivation of the species or its population genetics, and/or Wollemi Pine research and recovery planning.
The Foundation for National Parks and Wildlife and the Foundation and Friends of the Botanic Gardens for donations early in the conservation program; the NSW Government’s Saving our Species program, which has been the primary source of funding for the conservation program since 2016; the NPWS Assets of Intergenerational Significance program for additional operational funding; and the National Geographic Society for its support of the translocation program via a 2021 Species Recovery Grant.
The neighbours of Wollemi National Park, who continue to assist this project in many ways.

Wollemia nobilis W.G. Jones, K.D. Hill & J.M. Allen (Wollemi Pine) is a large tree endemic to Wollemi National Park (WNP) in central eastern New South Wales. It is currently known from just one wild population of 45 mature individuals and 46 juveniles occurring across four discrete stands (Mackenzie et al. 2021; Mackenzie & Auld in press). Conservation of this single population is critical for the species’ persistence in the wild.
The Wollemi Pine is threatened by demographic and environmental stochasticity due to its extremely small population size and geographic distribution; high severity and high frequency fire; dieback from the pathogens Phytophthora cinnamomi and P. multivora; unauthorised site visitation resulting in trampling of seedlings and juveniles, soil compaction and erosion, introduction of novel pathogens and weeds, and spread of existing pathogens; disturbance by feral pigs and deer; and the effects of anthropogenic climate change, including changes to temperature, rainfall, and disturbance regimes (fires, floods, heatwaves, and storms) (NSW TSSC 2015).
This document is the third National Recovery Plan for the Wollemi Pine and has been prepared in accordance with the NSW Biodiversity Conservation Act 2016 and the Commonwealth Environment Protection and Biodiversity Conservation Act 1999. It describes the current conservation status, documents past and ongoing management actions, summarises current biological and ecological knowledge, and details the research and management actions necessary to halt decline and support recovery of the Wollemi Pine.
The Wollemi Pine was discovered in 1994 by NSW NPWS Officer David Noble who, whilst off-duty and canyoning in Wollemi National Park, found a single stand of Wollemi Pine consisting of 19 mature individuals within a remote rainforest gorge (Jones et al. 1995; Mackenzie et al. 2021; Mackenzie & Auld in press). The significance of this rare species, which belongs to the 200-million-year-old Araucariaceae family, was quickly recognised, and the Wollemi Pine was subsequently listed as Endangered in NSW in 1995 due to its restricted distribution and small population size. In 1998, the species was also listed as Endangered by the Commonwealth and the first recovery plan for the Wollemi Pine was released, produced by the Wollemi Pine Recovery Team (Nash & Ravallion 1998).
Due to the rarity of the Wollemi Pine and the fragility of the species and its habitat, the exact location of the wild population has never been publicly released. The Wollemi Pine Access Strategy, which was first implemented under the 1998 recovery plan, remains in place to this day and restricts access to the wild stands to the minimum essential staff (Nash & Ravallion 1998; NSW DEC 2006a). However, public interest in the species has remained strong due to the species’ rarity, the history of its discovery and its connection to the age of the dinosaurs. Therefore, in 2005, a commercialisation strategy was launched to release Wollemi Pine cuttings to the public for sale and propagation. The purpose of this strategy was primarily to reduce the risk of damage to the wild population through illegal collection. Commercialisation has also increased community engagement in the conservation of the species, increased its prevalence in international botanic gardens, and has raised funds through royalties for the species conservation (NSW DEC 2006a).
In 2005, Phytophthora cinnamomi, a water-borne mould, was detected within Stand 1 of the wild Wollemi Pine population, representing a significant new threat to the species. It is thought that the mould was brought into the stand by unauthorised visitors via contaminated footwear or equipment (Mackenzie et al. 2021). Following this discovery, a second, updated recovery plan for the species was published in 2006 (NSW DEC 2006a) and adopted under the EPBC Act in 2007. Under the 2006 recovery plan, the establishment of new Wollemi Pine stands using translocation was prioritised to improve the long-term viability of the species in the wild by increasing its population size and geographic range, and thereby reducing its risk of extinction due to stochastic events (NSW DEC 2006a). In 2012, a pilot experimental translocation site was established off-park, on land managed by BMBGMT, with the goal of learning what was required for successful Wollemi Pine translocation into more remote areas (Zimmer et al. 2016). During this time, the Wollemi Pine was also uplisted to Critically Endangered in NSW in 2015 and nationally in 2018, in part due to an inferred projected decline in population size caused by the threat of dieback from Phytophthora infection (NSW TSSC 2015; Cwlth TSSC 2018).
The results of the 2012 pilot translocation informed two remote-area translocations into WNP conducted in 2019, with the long-term goal of increasing the size and geographic distribution of the wild population, although translocated stands will only be considered part of the wild population once they have produced second-generation seedlings and are self-sustaining (Mackenzie 2021; Mackenzie et al. 2021). Unfortunately, during Australia’s catastrophic 2019–20 ‘Black Summer’ bushfire season, the wild Wollemi Pine population and all three translocated stands were heavily impacted by fire. The impacts of these fires on the wild Wollemi Pine population were mitigated by an intensive, multi-agency fire suppression effort which involved the installation of irrigation systems, active water bombing, laying of fire retardant around the catchment, and active fire suppression by remote-area firefighters. This nationally recognised emergency effort likely limited the severity of the blaze to understory fires which primarily impacted juvenile plants (Mackenzie et al. 2021). The impacts of the 2019–20 fires, the early success of the three translocations, the species’ up-listing to Critically Endangered, and a review of the previous recovery plan (Appendix 2: Evaluation of progress towards objectives from previous recovery plan) all provided the impetus for the development of this updated recovery plan.
Conservation status
The Wollemi Pine is listed as a Critically Endangered species under both the NSW Biodiversity Conservation Act 2016 (BC Act) (NSW TSSC 2015) and the Commonwealth Environment Protection Biodiversity Conservation Act 1999 (EPBC Act) (Cwlth TSSC 2018). The species is also listed as Critically Endangered on the IUCN Red List of Threatened Species (Thomas 2011; Mackenzie & Auld in press).
The species is listed under Criteria B1ab (iii) (v), B2ab (iii) (v) and C2a (ii) under the EPBC Act. The main reasons for the species’ eligibility are:
- A highly restricted geographic range with an area of occupancy and extent of occurrence of 4 km2 AND known to exist at only one location AND continuing decline inferred in geographic distribution (extent of occurrence and area of occupancy), area and extent and quality of habitat, and the number of mature individuals.
- Fewer than 250 mature plants known AND 90-100% of individuals in one population.
Additionally, in 2021, 5,000 ha of land surrounding the wild Wollemi Pine population was declared as an Asset of Intergenerational Significance (AIS) under the amended NSW National Parks and Wildlife Act 1974 (NPW Act) (NSW NPWS 2022; 2023). This same area is also registered as an Area of Outstanding Biodiversity Value (AOBV) under the BC Act, having been previously declared as Critical Habitat under the repealed NSW Threatened Species Conservation Act 1995 (NSW DEC 2006b).
The Wollemi Pine is identified as one of 110 priority species under the Australian Government’s Threatened Species Action Plan 2022–2032 and the Greater Blue Mountains is identified as one of 20 priority places (DCCEEW 2022).
Recovery planning
Commonwealth
The EPBC Act allows for the development of a recovery plan to provide for the research and management actions necessary to stop the decline and support the recovery of a listed threatened species, so that its chances of long‑term survival in nature are maximised. This document has been drafted in partnership with the Wollemi Pine Recovery Team and replaces the previous recovery plan for the Wollemi Pine (NSW DEC 2006a).
NSW
The BC Act provides for the development of strategies to promote the conservation of threatened species and threatened ecological communities. The BC Act specifically requires the NSW Environment Agency Head to establish a Biodiversity Conservation Program delivering strategies, actions, monitoring and reporting to maximise the long-term security of threatened species and threatened ecological communities in nature, and to minimise the impacts of Key Threatening Processes on biodiversity and ecological integrity. In NSW, Saving our Species (SoS) is the adopted program that fulfills these requirements. This recovery plan has been developed to be consistent with the NSW SoS program; ongoing strategies and actions for the conservation of the Wollemi Pine under the SoS program will be informed by this plan.
The NSW NPW Act allows the NSW Minister for the Environment to declare any area of exceptional value within a national park as an Asset of Intergenerational Significance (AIS). A 5,000 hectare area of the catchment surrounding the wild Wollemi Pine population was declared as an AIS in 2021, recognising the area as important habitat for the Wollemi Pine. In accordance with the obligations of the National Parks and Wildlife Regulation 2019, a Conservation Action Plan (CAP) was developed in 2021 (NSW DPE 2022). NPWS has a statutory obligation to implement this CAP. The CAP identifies environmental values of the land, the key risks to those values, management activities to address and mitigate the risks, and requirements to measure and report on the condition of the AIS.
The known wild population of the Wollemi Pine occurs entirely within Wollemi National Park, which is managed by the NSW NPWS. The security of the Wollemi Pine population is aided by National Park tenure and is governed by the provisions of the NSW NPW Act as well as amendments to the Act which allowed for the declaration of the Wollemi Pine habitat as an AIS. These provisions offer a strong level of legal protection to the Wollemi Pine against activities which could negatively impact the species. Additionally, Commonwealth and NSW legislation detail assessment and approval pathways for activities that may impact on listed threatened species such as the Wollemi Pine.
NSW NPWS are responsible for the assessment and approval of all actions under this plan via the appropriate NSW and Commonwealth pathways. Where required, these processes will be completed prior to commencement of any action.
Commonwealth process
The EPBC Act additionally regulates actions that may result in significant impacts to Matters of National Environmental Significance (MNES). It is an offence to undertake any such actions in areas under State or Territory jurisdiction, as well as in Commonwealth-owned areas, without obtaining prior approval from the Federal Minister for the Environment. As the Wollemi Pine is listed nationally under the EPBC Act, any person proposing to undertake actions likely to have a significant impact on this species must refer the action to the Federal Minister for the Environment for consideration. The Minister must then decide whether the action requires EPBC Act approval.
Matters of National Environmental Significance relevant to this plan include:
a listed threated species, the Wollemi Pine
the Greater Blue Mountains World Heritage Area
the Greater Blue Mountains National Heritage Place.
NSW process
Within NSW, the Environmental Planning and Assessment Act 1979 defines the primary pathway for assessment and approval of activities. This includes activities related to the management of a threatened species and its habitats. The obligations of the Act are extended though additional NSW legislation including, but not limited to:
Biodiversity Conservation Act 2016
Fisheries Management Act 1994
Heritage Act 1977
National Parks and Wildlife Act 1974
Native Title Act 1993
Rural Fires Act 1997
State Environmental Planning Policy (Transport and Infrastructure) 2021
Wilderness Act 1987.
Key Threatening Processes
Both the BC Act and the EPBC Act provide for the identification and listing of Key Threatening Processes (KTPs). A KTP is a process that threatens, or has the capability to threaten, the survival or evolutionary development of species, populations or endangered ecological communities. The listing of KTPs does not regulate or prevent actions directly.
Three KTPs currently listed under the BC Act are known to threaten the Wollemi Pine. These are:
anthropogenic climate change
infection of native plants by Phytophthora cinnamomi
high frequency fire resulting in the disruption of life cycle processes in plants and animal and loss of vegetation structure and composition.
Three KTPs listed under the EPBC Act are known to threaten the Wollemi Pine. These are:
loss of climatic habitat caused by anthropogenic emissions of greenhouse gases
dieback caused by the root-rot water mould (Phytophthora cinnamomi)
fire regimes that cause declines in biodiversity.
In addition to these listed KTPs, a Threat Abatement Plan (TAP) for P. cinnamomi is in place under the EPBC Act (DoEE 2018). The TAP for P. cinnamomi establishes a national framework to guide and coordinate Australia’s response to the pathogen. This recovery plan contributes to the objectives of the TAP for P. cinnamomi by:
identifying the Wollemi Pine as a biodiversity asset affected by the pathogen and prioritising its protection
reducing the spread and mitigating the impacts of the pathogen to protect the Wollemi Pine
including the impact of the pathogen on the Wollemi Pine in public information to increase awareness of the pathogen.
The actions targeting P. cinnamomi in this recovery plan are consistent with those outlined in the TAP.
International obligations
Under the EPBC Act recovery plans should assist in the co-operative implementation of Australia’s international environmental responsibilities (Object E) and must have regard to meeting Australia’s obligations under international agreements. Australia is signatory to two international agreements relevant to this recovery plan:
United Nations Convention on Biological Diversity
World Heritage Convention.
Biological diversity
Under the Convention on Biological Diversity, all parties are required to have a National Biodiversity Strategy and Action Plan in place. This recovery plan has been prepared to be consistent with the current edition of this national plan, Australia’s Strategy for Nature 2024-2030 (Commonwealth of Australia 2024). This recovery plan contributes to the achievement of the objectives of the Strategy for Nature by:
contributing to the conservation management of Australia’s landscapes
setting out actions to protect and secure the Wollemi Pine in the wild
describing actions to reduce threats to the Wollemi Pine
increasing Australians’ understanding of the value of the Wollemi Pine
improving and sharing knowledge of the Wollemi Pine to aid decision making.
World heritage
The wild Wollemi Pine population and the two translocated stands exist within the Greater Blue Mountains World Heritage Area (GBMWHA), which was inscribed on the World Heritage List in November 2000. The area supports outstanding biodiversity values, including over 100 eucalypt species occurring in sclerophyll ecosystems in an extraordinarily diverse area rich in natural and cultural values. The Wollemi pine is listed in the Statement of Outstanding Universal Value under Criterion (ix): be outstanding examples representing significant on-going ecological and biological processes in the evolution and development of terrestrial, fresh water, coastal and marine ecosystems and communities of plants and animals (NSW NPWS 2000).
The GBMWHA Strategic Plan was developed in 2009 to assist in meeting Australia’s international responsibilities under the World Heritage Convention (NSW NPWS 2009). The plan ensures that appropriate consideration is given to the GBMWHA’s World Heritage values by managers when developing management prescriptions for the GBMWHA reserves, and that they are developed and implemented in a consistent and coordinated way. This recovery plan has been prepared to be consistent with the objectives of the Strategic Plan through:
conserving and transmitting to future generations the Wollemi Pine and its habitat as a key value of the GBMWHA
coordinating with existing plans including the NSW SoS program, the NPWS AIS CAP and the Wollemi National Park Plan of Management as part of an integrated planning program for the GBMWHA
giving the GBMWHA a function in the life of the Australian community by providing for the commercial propagation of the Wollemi Pine and planting of Wollemi Pine in botanic gardens (such as Botanic Gardens of Sydney, Australian Botanic Garden Mount Annan, and Blue Mountains Botanic Garden Mount Tomah)
strengthening appreciation and respect for the GBMWHA’s World Heritage values through educational programs surrounding the Wollemi Pine
taking the appropriate scientific, technical, legal, administrative and financial measures necessary for implementing these principles
providing for continuing community and technical input in managing the Wollemi Pine through collaborations with a diverse range of groups and organisations.
The previous recovery plan (NSW DEC 2006a) guided conservation management action for the Wollemi Pine, including the continuation of in situ ecological monitoring and site surveillance, expansion of the ex situ collection, establishment of translocated stands, and research into Wollemi Pine ecology and environmental tolerances. A detailed evaluation of the former recovery plan is included in Appendix 2. The current plan is informed by the previous recovery plans, with additional key considerations including the impacts of the 2019–20 bushfires, and expansion of the ex situ collections and translocation program. Key learnings since the previous recovery plan which have informed updated recovery actions include:
an improved understanding of the wild Wollemi Pine population structure and the fire response of different life history stages
the resolution of population monitoring that is required to detect change
the discovery of new wild individuals
an improved understanding of the genetic diversity of the wild Wollemi Pine population
the importance of establishing multiple ex situ collections to provide failsafe backups
the importance of considering recurrent disturbances such as fire and drought when conducting recovery actions (such as translocations).
Implementation of recovery planning is a collaborative effort involving a range of stakeholders including:
NSW Department of Climate Change, Energy, the Environment and Water (NSW DCCEEW), including NSW National Parks and Wildlife Service (NPWS)
Royal Botanic Gardens and Domain Trust (RBGDT)
Australian Government, including the Commonwealth Department of Climate Change, Energy, the Environment and Water (Cwlth DCCEEW)
universities and other research organisations.
These organisations are responsible for and/or involved in the protection, rehabilitation and management of Wollemi Pine and its habitat, including via public education, survey, research and monitoring. NSW NPWS have statutory responsibility for the protection of the Wollemi Pine in WNP.
Other groups that may be involved in the implementation of recovery actions that may also be affected by this recovery plan, include:
Traditional Owners and Indigenous Land Councils
local government councils
commercial horticultural groups
tourism organisations operating in WNP
private individuals using WNP recreationally.
Wollemi National Park contains an extensive representation of Aboriginal sites and places with links to traditional and spiritual life. The inter-connection between places and stories within the park are a significant part of regional Aboriginal cultural identity. The park also occurs at the junction of Land Council boundaries, including the Wanaruah, Bathurst, Deerubbin and Metropolitan Local Aboriginal Land Councils (LALCs) and is partly included in a Native Title claim (Warrabinga Wiradjuri #7 (NC2018/002)). There is a recognised need for free, prior and informed consent from Aboriginal interest groups regarding WNP land management matters, including the management of the Wollemi Pine.
The on-going involvement of the Aboriginal community with the management of WNP is encouraged and supported by NPWS at multiple levels. In early 2024, a series of facilitated workshops were held with Aboriginal interest groups and Traditional Owners of the GBMWHA as part of the development of a Strategic Plan for the GBMWHA (NSW NPWS 2024). The GBMWHA property spans the Country of the Darkinjung, Dharawal, Dharug, Gungungurra, Wiradjuri and Wonnarua peoples and includes WNP, which is the largest reserve in the World Heritage Area. Furthermore, NPWS are considering options for models of Aboriginal joint management for national parks across NSW, including WNP (NSW DCCEEW 2024). As part of the ongoing development of this joint management model, targeted consultation has been undertaken in 2022 and 2023 with Traditional Owners and Aboriginal interest groups across NSW. The Blue Mountains Branch of NPWS works to establish and maintain relationships with Aboriginal stakeholders to facilitate ongoing engagement on the natural and cultural heritage values of WNP. Additionally, the Aboriginal Park Partnerships Program has funded a cooperative program with the North East Wiradjuri Company to undertake protection of Aboriginal sites within Wollemi National Park.
Ensuring that Wollemi Pine land management issues are included, where appropriate, in these ongoing consultation processes with Aboriginal stakeholders is an important aim of this recovery plan. NPWS Blue Mountains Branch will continue to maintain relationships with Aboriginal stakeholders to ensure consultation can occur as necessary, especially when management may impact on the Aboriginal natural and cultural heritage values of the park.
Ecological
The history of the discovery of the Wollemi Pine highlights the importance of habitat conservation and the integral role that national parks play in the conservation of biodiversity. It also demonstrates the importance of conserving areas of diverse vegetation types and the crucial role that refugia locations (like the canyon habitat the Wollemi Pine occupies) play in the environment by providing relatively stable conditions through periods of great climatic change (Hill 1995).
The wild population of Wollemi Pine exists within warm temperate rainforest, among species typical of the canyons and gorges of the Blue Mountains region. This recovery plan encourages the study and conservation of the species and its habitat that will directly benefit local species and communities. Numerous EPBC Act-listed species occur across the Blue Mountains region and may benefit from the actions detailed in this recovery plan, although significant benefit or impact for any one co-occurring listed species is not expected. It is hoped that findings from this work will also provide insights to improve management of similar ecosystems and unique species across NSW.
The Wollemi Pine is a flagship species for conservation. People are inspired by the story of its discovery and its connection with the age of the dinosaurs. These stories raise interest and awareness of threatened plant conservation and biodiversity conservation in general. The world-wide concern for the fate of the Wollemi Pine during the 2019–20 bushfires demonstrated its global appeal.
Since the commercial release of the species commenced in 2005, Wollemi Pine has continued to be keenly sought after as a horticultural plant. A proportion of the profit from sales was initially directed to various conservation activities, however the commercial partnership ceased in 2010 and royalties no longer contribute to conservation activities (B. Summerell, pers. comm).
Ecotourism opportunities have been created in NSW through the planting of Wollemi Pine in botanic gardens, such as BGOS, ABGMA, and BMBGMT. The Wollemi Pine has contributed to the public perception that NSW has unique biodiversity and high conservation values and is a destination for tourists wanting to experience the Australian natural environment.
The social effects of controlling impacts on the wild Wollemi Pine stands (including restricting visitation) are minimal as the species’ habitat is reserved within a national park. It is noted that the declaration of the Wollemi Pine habitat as an Area of Outstanding Biodiversity Value and associated regulations apply to 1% of Wollemi National Park. The RBGDT and others have created opportunities for the public to view and appreciate the species by establishing plants in a variety of local, national and international public gardens. Through the commercial partnership, plants have also been made available for private horticulture.

The Wollemi Pine is of significant scientific interest because of its long evolutionary history and its phylogenetic distinctiveness. Despite the common name ‘pine’, the species does not belong to the Pinus genus, nor to the pine family Pinaceae. Instead, it belongs to the ancient conifer family Araucariaceae, which evolved approximately 210 million years ago, and Wollemi nobilis is the sole extant species within the genus, Wollemia.
Wollemia
Wollemia was confirmed to be a distinct genus by Gilmore and Hill (1997) through DNA sequencing of the plastid gene rbcL. This sequence data combined with different ranges of other conifer taxa suggests that Wollemia is ancestral to Agathis and Araucaria and may be the earliest derived genus in Araucariaceae (Setoguchi et al. 1998) or, alternatively, that Wollemia is a sister group to Agathis, with these two forming a clade that is sister to Araucaria (Gilmore and Hill 1997; Stefanović et al. 1998). Analysis by Liu et al. (2009) supported Wollemia being a sister group to Agathis. The most recent study concluded Agathis and Wollemia formed a separate clade to Araucaria that was distinct by the Cretaceous or Palaeogene period (Escapa & Catalano 2013).
Evolutionary history and fossils
The Wollemi Pine is of considerable significance in the study of the evolutionary relationships of early Gondwanan flora, and the species has contributed to the understanding of structures in fossil Araucariaceae (Macphail et al. 1995; Chambers et al. 1998; Dettmann & Jarzen 2000). Fossil evidence suggests that major gymnospermous (non-flowering) forests or woodland strata were once widespread in Australia, and Araucariaceae fossils from the Tertiary period have been found in every Australian state (Lange 1982). A decline in the distribution of Araucariaceae occurred in the late Quaternary, coinciding with increasing temperatures, dryness and incidence of fire (Kershaw and Wagstaff 2011). Understanding of the prehistoric distribution of the Wollemi Pine has previously been informed by the distribution of the fossil pollen Dilwynites, understood to be very similar to the pollen of Wollemi Pine. Dilwynites pollen has been identified in sediments from Australia, New Zealand, Antarctica, and Patagonia, from the late Cretaceous to the late Tertiary (Macphail et al. 1995; Chambers et al. 1998; Dettmann & Jarzen 2000; McLoughlin & Vajda 2005; Macphail et al. 2013). However, more recent analyses (Macphail & Carpenter 2014; Seyfullah et al. 2023) have demonstrated that Dilwynites pollen may represent other members of the Araucariaceae, in addition to Wollemi Pine, and that Wollemi Pine pollen is more variable than had previously been reported.
The Wollemi Pine is a long-lived, monoecious conifer which can grow in excess of 40 metres in height (NSW DEC 2006a); however, the mean height of adult trees in the wild population is presently c. 20 m (Mackenzie, Clarke and Auld, unpubl. data). The Wollemi Pine has an unusual growth habit of reiteration, whereby vertical branches (with their own lateral branches) develop from epicormic shoots on the trunk and partially reiterate the architecture of the young adult plant, and new trunks develop via coppicing from the base of the tree (Figure 1) (Hill 1997). This pattern of architecture and reiteration is unlike any other extant Araucariaceae species and is possibly unique among all plants (Mackenzie et al. 2021). Individual trunks range up to 90 cm in diameter at breast height (DBH). There are very few single-stemmed trees in the wild population; instead, mature trees are usually multi-trunked and may comprise more than 40 stems greater than or equal to 2 m tall and/or with a DBH greater than or equal to 2 cm, in addition to numerous smaller coppice stems (Mackenzie, Clarke and Auld, unpubl. data).
Figure 1 Self-coppicing habit of Wollemi Pine

Source: © Copyright, Patricia Meagher, Royal Botanic Gardens and Domain Trust
Primary branches are plagiotropic (grow laterally) and are short lived and cleanly abscising (Hill 1995; Hill 1997). New primary branches are produced on epicormic shoots from the trunk which creates a branched crown that is slender and columnar (Hill 1997; Burrows et al. 2003). The Wollemi Pine produces three different types of foliage depending on its position on the tree (along the stem, on juvenile branches, or on mature branches; see Growth habit under section 2.5). Leaves range from 3–80 mm in length and 2–8 mm in width and tend towards a linear shape but can be narrowly oblong or narrowly triangular (Jones et al. 1995). Depending on the type of shoot, leaves can be arranged helically or opposite to subopposite in rows of two (juvenile branches) or four (mature branches). The bark on younger stems peels in thin, fragile, equidimensional dark red-brown scales, while the bark on older trunks becomes densely covered with soft and spongy nodules up to 10 mm in diameter and 15 mm in length, forming a layer up to 20 mm deep (Jones et al. 1995).
The Wollemi Pine is monoecious (male and female reproductive organs are borne on the same individual). Reproductive organs are arranged in cones or cone-like structures called strobili (Harden 1990), with male cones developing before female cones. Male cones are cylindrical, up to 153 mm long and 25 mm wide, and turn from green to dark red-brown as they mature (Figure 2). At maturity they can bear more than 500 scales which are helically arranged (Jones et al. 1995). Each scale has 4–9 microsporangia in which the oval-shaped, granular, unwinged pollen is formed (Jones et al. 1995). Female cones are spiny, globular to broadly egg-shaped, 125 mm long and 100 mm in diameter. Each cone has between 250 to 300 bract-scales flattened, with a lateral wing, which turn from mid-green to brown as they mature (Figure 3). Seeds are produced in the female cones and have a single wing, are flat, light brown, 7–11 mm long and 5–9 mm wide including the wing (Jones et al. 1995).
Figure 2 Male cone of Wollemi Pine

Source: © Copyright, Jaime Plaza, Royal Botanic Gardens and Domain Trust
Figure 3 Female cone of Wollemi Pine

Source: © Copyright, Jaime Plaza, Royal Botanic Gardens and Domain Trust
Wollemi Pine is a relict species currently known from just a single wild population, which occurs as four discrete stands in a single catchment in Wollemi National Park on the Central Tablelands of New South Wales in southeastern Australia (Figure 4). These four stands, along with any as-yet undiscovered wild stands in Wollemi National Park, are all important populations as defined by the EPBC Act, that are necessary for the long-term survival and recovery of the Wollemi Pine.
The Wollemi Pine has a very highly restricted geographic distribution; both the extent of occurrence and area of occupancy (sensu IUCN Standards and Petitions Committee 2024) are only 4 km2 each. Extensive ground and helicopter searches of the Wollemi Pine catchment and adjacent areas since the species’ discovery have not found any additional stands. The exact location of the wild Wollemi Pine population has never been publicly released due to the risk of damage to the species and its habitat from unauthorised visitation and/or illegal collection.
The historic decline of the Wollemi Pine is likely to have occurred alongside the decline of other rainforest flora in Australia, with the climate becoming increasingly dry into the late Quaternary (Kershaw and Wagstaff 2011). Hence, the rarity of the Wollemi Pine, as distinct from many other threatened plant species, is not attributed to human impact, rather to natural changes in climate over a geological time scale.
Figure 4 Location of Wollemi National Park, New South Wales, Australia.
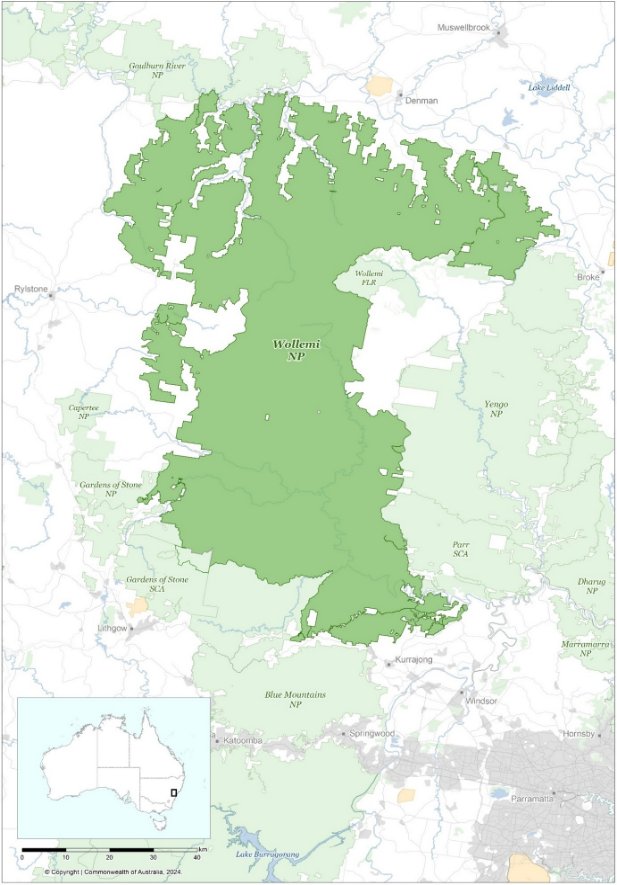
Source: Base map Geoscience Australia.
Wild population structure
The prolific coppicing habit of wild Wollemi Pine makes it difficult to delineate genetically distinct individuals in the field. In lieu of completed population genomic analyses, putative individuals have been defined as discrete clumps of stems or trunks inferred to share a common base. The wild population is composed of adult, juvenile and functionally juvenile individuals. Male pollen cones develop on juvenile trees several years before the first female seed cones, and individuals are regarded as adults once they produce female seed cones. Adult trees that suffer complete loss of their mature trunks after fire, windthrow or impacts from falling rocks and trees, may enter a secondary juvenile phase while replacement stems resprout and mature (Mackenzie & Auld, in press). Such functionally juvenile individuals are expected to have higher survival and faster growth and maturation than true juveniles (those yet to produce a mature trunk since establishing from seed) due to well-developed adult root systems in the former (Mackenzie & Auld, in press).
A comprehensive demographic inventory of the wild population was undertaken in 2020 and 2021 (after the 2019–20 bushfire season). This entailed updated assessments of the boundaries between putative individuals and their reproductive status (adult, juvenile or functionally juvenile) (Mackenzie & Auld, in press). In addition, a number of previously undetected juvenile and adult trees were identified on high cliffs adjacent to known stands(Mackenzie et al. unpubl. data).
As of June 2024, 45 adult trees remain in the wild with 19 of these in the largest stand (Table 1; Mackenzie & Auld, in press). The population includes a further 34 true juveniles and 12 functional juveniles; however, 47% and 75% of these, respectively, were completely top-killed in the 2019–20 fires and currently persist as vulnerable basal resprouts (Mackenzie, Clarke and Auld, unpubl. data). A further 113–183 immature individuals (seedlings, juveniles and functional juveniles) were killed in the fires including 92–95% of the pre-fire bank of smaller juveniles (those < 2m tall), among which were many individuals over 20 years old, with some up to 60 years old (Mackenzie, Clarke and Auld, unpubl. data). This small juvenile bank is expected to re-establish over the next 20–30 years via ongoing annual seed production in adult trees (Mackenzie et al. 2021); However, the losses of larger juveniles will take many more decades to recover. The first systematic post-fire seedling surveys are scheduled for summer 2024-25 (B. Mackenzie, pers. comm.).
Table 1 Wollemia nobilis post-fire population structure
Stand | No. adults | No. f. juv | No. juv | Total |
1 | 19 (15-23) | 1 (1-2) | 8 (7-10) | 28 (23-35) |
2 | 11 (10-12) | 8 (8-8) | 17 (17-19) | 36 (35-39) |
3 | 2 (2-2) | 1 (1-1) | 1 (0-2) | 4 (3-5) |
4 | 13 (12-13) | 2 (2-1)A | 8 (8-11) | 23 (22-25) |
Total | 45 (39-50) | 12 (12-12) | 34 (32-42) | 91 (83-104) |
Note: Data are best estimate (lower bound-upper bound), reflecting uncertainty in the exact number of genetic individuals and their reproductive status. f. juv – functional juveniles; juv – true juveniles. A The lower bound is higher, as an individual comprising three juvenile ramets, has been interpreted as a single functional Juvenile in the lower bound and as three juveniles (and therefor one less functional juvenile) in the upper bound.
Source: Reproduced in full from the latest Wollemi Pine IUCN Red List Assessment (Mackenzie & Auld, in press).
Translocations
In addition to the wild population, three translocated stands of Wollemi Pine have been established, as described below.
Mount Tomah stand
In 2012, 191 Wollemi Pine cuttings of varying size, age and origin were planted in different microsites, from deep in rainforest to woodland ecotones in an area of natural forest near BMBGMT (Zimmer et al. 2016). This semi-wild, off-park stand was maintained as an experimental translocation with the dual aims of learning what is required for successful Wollemi Pine translocation and testing the effect of available light on Wollemi Pine growth and survival. Prior to the 2019–20 bushfire season, survival of translocated individuals was high (80%); however, the entire stand was impacted by the fires and had declined to just 28 resprouting individuals (18% of the pre-fire population) as of May 2024 (Allan & Allan, unpubl. data) (Figure 5). With the focus of the translocation program now on two remote-area stands in Wollemi National Park, the role of the Mount Tomah stand in the conservation program is being re-evaluated and it is likely to be maintained as a research and/or educational site.
Wollemi National Park stands
Following the success of the 2012 pilot translocation, two remote-area translocated stands were established in WNP in mid-2019, in deep, steep sided rainforest canyons with perennial watercourses (Mackenzie et al. 2021). Known respectively as T1 and T2, the aims of these translocations are to:
use an adaptive management framework to experimentally address uncertainty in the optimal planting strategy to accelerate maturation and maximise longevity of translocated Wollemi Pine saplings (specifically, where to position saplings along gradients in vegetation, available light, fire and drought risk, and which type of source material – cuttings or seed-grown plants – to use)
address knowledge gaps in the ecology of Wollemi Pine (including its persistence and regeneration niches) that are relevant to conservation management of the wild population
ultimately, to increase the size and geographic distribution of the wild population (which requires the translocated stands to first become self-sustaining and is indicated by the appearance of second-generation seedlings).
These plantings extended the learnings from the 2012 pilot translocation and incorporated advances in propagation techniques and population genomics to produce large numbers of high quality and genetically diverse Wollemi Pine saplings. Additionally, the ecological experiments underlying the WNP translocations explicitly address the effects of disturbance regimes such as fire and drought on trade-offs between longevity, growth and lifetime reproductive output of translocated saplings over different timescales (Mackenzie 2021; Mackenzie et al. 2021).
Both T1 and T2 had an initial population size of 218 saplings each (Figure 5); however, they were heavily impacted by the 2019-20 fires soon after establishment and before the saplings had time to develop fire resistance. No saplings resprouted successfully post-fire at either site but 64 (30%) and 6 (3%) saplings escaped burning at T1 and T2, respectively.
In 2021 both T1 and T2 were augmented with c. 250 saplings each (Mackenzie et al., 2021), although 25% of the total population at T2 was impacted by a landslide in 2022, resulting in 13% mortality. As of May 2024, from the original 2019 planting, only 53 (24% pre-fire population) and 3 (1% pre-fire population) individuals persisted at T1 and T2, respectively (Mackenzie et al., unpubl. data). Survival of the 2021 augmentation cohort remains high, attributable in part to three years of favourable La Niña conditions from 2021 to 2023, with 210 (84%) saplings persisting at T1 and 187 (75%) saplings persisting at T2 as of May 2024 (Mackenzie et al., unpubl. data). These populations were augmented with c. 180 saplings each in April and June of 2024, bringing the total population size of T1 to 456 saplings and that of T2 to 379 saplings (Figure 5).
Figure 5 Population size of translocated stands
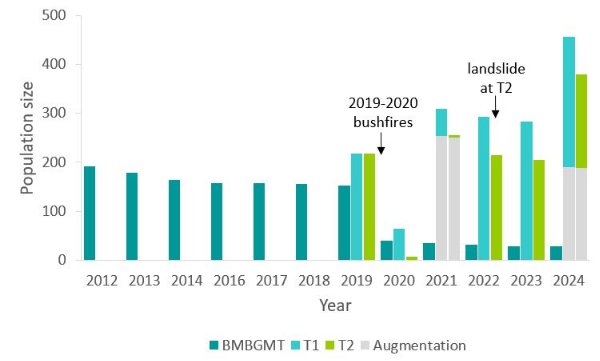
Source: Mackenzie et al. (2021) Figure 3a, updated with 2022, 2023 and 2024 monitoring data.
Ex situ collections
The translocation program is supported by an active ex situ conservation program which centres around a potted living collection and seed bank maintained at the Australian PlantBank at ABGMA. The bulk of the living collection consists of plants produced by vegetative propagation using material collected from wild individuals and currently captures 60% of the known genets in the extant wild population that are currently large enough to collect propagation material from (Mackenzie et al. 2021; Mackenzie et al. unpubl. data). Many of the wild individuals yet to be incorporated into the collection have only recently been discovered and/or accessed by field teams and are in a vulnerable state of post-fire recovery (Mackenzie et al. 2021).
From 2020-21, all known extant individuals in the wild Wollemi Pine population were sampled for their DNA, with the exception of two newly discovered juveniles in Stand 2 (B. Mackenzie pers. comm.). Several recent collections of wild seeds have also been made, the largest of which was collected by helicopter in 2016 and consists of over 500 seeds from Stand 1 and over 100 seeds from Stand 4. These are held in the seed bank at the Australian PlantBank. Seed collection remains problematic due to the height of the cones on the trees and, therefore, most seeds have been collected by helicopter. Good seed crops are reported for many cultivated plants, but their genetic diversity is low due to the limited number of genotypes initially propagated for commercial release.
Work is underway to create a comprehensive in-ground, cooler climate backup of the ABGMA potted collection at BMBGMT, and a smaller living collection is maintained at BGOS. To provide maximum climate change resilience, a genetically diverse metacollection (Griffith et al. 2020) of Wollemi Pine is being developed by the RBGDT, BGCI, Forestry England, and various national and international partner agencies. To date, genetically diverse samples of plants have been distributed under agreement to botanic gardens and arboreta in Australia, Europe, the UK and the USA.

The vegetation communities in which the Wollemi Pine occurs were described by Benson and Allen (2006) and are summarised here. Most Wollemi Pines grow in warm temperate rainforest within deep sandstone gorges and gullies, while a few individuals grow on high ledges where they mix with dry sclerophyll vegetation. All four Wollemi Pine stands are characterised by a closed canopy vegetation structure with an understorey of shrubs or juvenile trees, and a dense layer of ferns. Vines are common in all of the stands. The dominant trees are Wollemi Pine, Ceratopetalum apetalum (coachwood), Doryphora sassafras (sassafras) and Syzygium smithii (lilly pilly). Eucalypt species, including E. piperita (Sydney peppermint), E. punctata (grey gum) and Angophora costata (smooth barked apple) occur in small numbers.
Geology and soil characteristics
The gorge walls are composed of Triassic sandstones from the Narrabeen Group. Soils are sandstone-derived boulder alluvium, with high organic matter and some shale component (Jones et al. 1995). The soil is very shallow. In some areas there is little or no soil layer. Roots of Wollemi Pine grow into rock fissures or extend for tens of metres away from the main groups of trunks. Nutrient levels are low and the soil is extremely acidic, often in the range pH 3–4, with low levels of most elements although high in aluminium, sulphate and iron (Offord et al. 2014; Zimmer et al. 2016 S2 Table). There are patches of highly saline soil (Offord et al. 1996; Offord unpubl. data).
Senescent Wollemi Pine branches fall and contribute substantially to the litter layer (Hill 1995). Decomposition of these fallen branches may contribute to the low pH of the soil. Acidity has not been found to inhibit growth of potted seedlings; indeed, potted plants exhibit higher growth rates in pH 4.5 soil compared to pH 6.5 soil (Offord et al. 2014).
Climate
The climate is typical of locations in the low altitude areas of the Blue Mountains. Air temperature data was recorded in Stand 1 for one year (1997–98) and showed that the highest average air temperatures occur in the stand from November to February with a maximum average of c. 30°C. In winter (June to August), the maximum average temperature ranges from 10–15°C with average minima near freezing (Offord and Meagher 2001).
Habitat dynamics and disturbance
The rainforests of the deep sandstone gorges are relatively protected from fire and drought compared to the surrounding woodlands and forests which occur on the ridges above the gorges. For this reason the habitat of the Wollemi Pine is sometimes referred to as a refuge or refugium. Disturbance regimes are also key to Wollemi Pine habitat (see Responses to disturbance regimes section).
Habitat critical to the survival of the species
Habitat critical to the survival of the Wollemi Pine is equivalent to the Wollemi Pine declared Area of Outstanding Biodiversity (AOBV) (NSW DEC 2006b), approximately 5,000 ha of the 500,000 ha Wollemi National Park which is registered under the BC Act. This area provides for natural range extension of the wild population and contains suitable habitat that is necessary for the ongoing survival and recovery of the species. Factors including topography and drainage as well as adjacent land use were considered in determining the extent of the area.
Significant advances have been made in our understanding of the biology and ecology of the Wollemi Pine since the publication of the previous recovery plan, particularly regarding population dynamics, seedling and juvenile growth and survivorship, responses to fire and drought, and translocation requirements.
Growth habit
Wollemi Pine produces three types of shoots:
Juvenile and lower canopy plagiotropic shoots: this type of shoot forms the lateral branches on juvenile Wollemi Pine or in the lower canopy on adult trees. This shoot is horizontal, and leaves begin as short scale-like leaves only 3 mm long but grow to 20–80 mm long and 2–5 mm wide. Leaves are linear to narrowly triangular, opposite or subopposite and grow in two opposite ranks (Jones et al. 1995).
Adult orthotropic shoots: this type of shoot grows vertically and includes the primary trunk and additional, subsidiary vertical shoots which develop from the trunk or adventitiously arise from just below the cotyledons (Burrows et al. 2003). These shoots exhibit a helical arrangement of leaves, which are small, only 3–10 mm long and 2–4 mm wide at the base, taper to an acute angle at the tip, have a sharp point and are narrowly triangular (Jones et al. 1995). Primary branches arise at the apex of these vertical shoots and are plagiotropic (lateral) and short-lived.
Adult upper canopy plagiotropic shoots: this type of shoot forms the lateral branches of adult Wollemi Pine, primarily in the upper canopy. Initially these branches grow nearly vertically, before becoming horizontal and later pendulous. Leaves begin as short scale-like leaves only 3 mm long but increase to 10–40 mm long and 4–8 mm wide (Jones et al. 1995). Leaves are narrowly oblong, opposite or subopposite decussate (each opposite pair occurs at right angles to the previous pair) and longer leaves are twisted at the base, giving the appearance of four flattened ranks of leaves (Jones et al. 1995; Chambers et al. 1998). Male (pollen) and female (seed) cones can develop at the tip of these branches when the branches are between 1 and 10 years of age. Typically, female cones occur on higher branches of the same trunk than males, which is thought to promote greater outcrossing (Jones et al. 1995, Hill 1997, Offord et al. 1999). After initially bearing cones, branches may continue to grow vegetatively and produce further cones. Branches grow for up to 11 years before cleanly abscising (Burrows et al. 2007).
The combination of these three shoot types, along with regular self-coppicing, from both the base and epicormically in undamaged individuals—an unusual trait for a member of the Araucariaceae family—contribute to the Wollemi Pine’s unique growth habit (Burrows et al. 2003).
Vegetative regeneration
The Wollemi Pine is capable of vegetative regeneration (re-sprouting) which occurs through undifferentiated, actively dividing plant tissues (meristems). These are carried in the upper axils between vertical shoots and their leaves and slowly develop into young buds (primordia) within the thickening bark. This is an unusual characteristic in conifers which are usually devoid of bud-forming potential (Burrows 1999, Burrows et al. 2003). These buds can replace the leading shoot if it is damaged but may also spontaneously develop into secondary orthotropic shoots. New shoots may also arise from the basal region possibly through adventitious bud formation, but this form of vegetative regeneration is poorly understood (Burrows et al. 2003). It appears that this unusual long-lived meristematic potential exists in most (if not all) leaf axils and many will develop into bud primordia. This slow but continued development provides a ready source of additional or replacement leaders and thus new branches and leaves. Basal coppicing can lead to a number of trunks of various ages on a mature tree.
Sexual reproduction
The Wollemi Pine is a gymnosperm with its reproductive organs borne on modified leaves called sporophylls, which are arranged in cone or cone-like structures called strobili (Harden 1990). Male cones appear in early to mid-summer and mature in spring when pollen is shed (Zimmer et al. 2015a). Female cones can first be observed in mid to late summer, and they are pollinated in the following spring. Fertilisation occurs approximately one year after pollination. Several months later, in late summer and early autumn, seed cones mature and female cones disintegrate, shedding their seed and individual bract-scales (Offord et al. 1999; Zimmer et al. 2015a; Prakash et al. unpubl. data).
Embryo development follows a similar pattern to Agathis australis (Owens et al. 1997), extending over almost 2 years from pollination to seed maturity. Interestingly, in Wollemia many ovules are destroyed by active pollen tubes (Prakash & Clarke unpubl. data). This may contribute to the relatively low rates of seed viability observed in Wollemi Pine—generally, less than 10% of the c. 250 ovules in female cones develop into viable seeds (Offord et al. 1999).
The Wollemi Pine is a monecious species (male and female cones occur on the same tree). Some level of self-pollination is likely to be occurring and may explain the production of seeds reported from single cultivated individuals. This may be contributing to inbreeding and the low diversity found in individuals stands (Stevenson et al. 2023 preprint). Seeds may also be produced by apomixis (asexual reproduction via seeds), although this is not known to occur in this family. The production of seeds follows a similar pattern to other members of the family, taking 18-24 months from the first emergence of female cones to seed maturity and cone dehiscence. Controlled studies on the breeding system of the Wollemi Pine are in progress at BMBGMT (Offord & Phelan, unpubl. data).
Seed dispersal and germination
Seeds of Wollemi Pine are relatively light compared to other gymnosperms (ranging from 10–44 mg; Meagher & Offord unpubl. data) and winged and are dispersed short distances within existing stands by gravity and wind. Aerial dispersal appears to be in a downslope direction as seedlings occur up to 30 metres downslope of the nearest tree but are more limited upslope, likely because of the high cliffs subtending most stands. It is possible that there may be very rare longer-distance movement of seeds by birds if they feed on cones and move the cones to another feeding tree. This could account for the current distribution of Wollemi Pine. There may also be movement of seeds downstream by water if floods coincide with seed fall (Offord et al. 1996). It appears likely that a proportion of seed is lost to predation. Crimson Rosellas (Platycercus elegans) have been observed foraging Wollemi Pine cones; a rat (Rattus rattus) skull has been found in one of the wild Wollemi Pine stands, indicating their presence (and potential to act as seed predators); and insect damage to seeds has also been observed (Offord et al. 1999).
For the first 20 years after the species’ discovery, the only source of cones and seeds for seed banking, propagation and research was the wild population. This has changed in recent years, as Wollemi Pines in cultivation have begun to mature and provide alternative sources of seed.
Laboratory trials suggest that seeds of Wollemi Pine germinate in the temperature range 24–30°C. However, the rate of germination is somewhat protracted unless the seeds are subjected to a period of cold moist stratification, at < 10°C, followed by incubation in the optimal range. This period of stratification is analogous to the temperature regime experienced after autumn seed fall, with seeds that survive winter germinating quickly in spring and early summer, presumably to take advantage of the moister conditions (Offord and Meagher 2001). In the field, seed emergence phenology is bimodal, with peaks in autumn and spring.
Seeds are orthodox or intermediate with regard to their storage capability; they can be dried down to less than 10% moisture content and stored at low temperature (-18°C) for up to five years, and possibly longer (C. Offord, pers. comm.). Wollemi Pine seeds contain around 40% oil, including a short chain omega-3 fatty acid not commonly found in plants. Seeds stored at sub-optimal temperatures (> 0°C) show decreased germinability and viability, which correlates with an increase in lipid degradation (Ng et al. 2024).
Seedling establishment and growth
Seedlings have been identified in the wild population by the presence of cotyledons and cotyledon scars. They occur in the wild on a variety of substrates including rocks, logs, tree ferns and in the soil litter layer (W. Jones, J. Allen, field obs.). Seedlings and juveniles occur in all four wild Wollemi Pine stands. It appears that the Wollemi Pine maintains a slow growing juvenile bank, as is common in rainforest trees, and other Araucariaceae (Zimmer et al. 2014). Seedlings germinate in most years, but most of these seedlings die during their first year. Potential explanations for this mortality include fungal attack and soil pathogens, adverse microclimates (too dry, too hot) and herbivory. However, once seedlings become established their survival rates are high, although growth rates are low.
Light mediates the growth of seedlings under glasshouse conditions, with best growth rates in cultivation between 25-75% ambient light (Offord et al. 2014). Glasshouse trials demonstrated increasing growth rates from 5%, 15% to 50% sunlight (Offord et al. 2014). Growth rates of seedlings in the wild are very slow, with less than < 1 cm growth in height per year (Zimmer et al. 2014), compared with up to 60 cm per year under glasshouse conditions (Offord et al. 1999) and in translocated seedlings (Mackenzie et al. unpubl. data). This can, to some extent, be attributed to light availability. However, Wollemi Pine can exhibit symptoms of photoinhibition (sunburn) and are susceptible to heat stress (Offord 2011). High growth rates of Wollemi Pine in cultivation suggest that Wollemi Pine seedlings in the wild are resource-limited by light, nutrients and rooting space. Increases in availability of these resources may coincide with canopy gap openings (such as via treefall) (Zimmer et al. 2014). The understorey of wild Stand 1 receives about 3% full sunlight and, in cultivation, increasing growth has been recorded in up to 50% full sunlight (Offord et al. 2014). Resource availability may play a role in limiting the transition from juvenile to mature tree, although fire return intervals are also likely to be key (Mackenzie et al., unpubl. data).
Mycorrhizal associations
Although mycorrhizal associations have been identified (McGee et al. 1999), as yet no mycorrhizal association appears necessary (symbiotic) for seedling growth and survival in the wild (Rigg et al. 2016a), or in cultivation (Offord et al. 1999).
Longevity and tree rings
The wood of the Wollemi Pine produces distinct and annual growth rings (Banks 2002) and, like other members of Araucariaceae, has alternate bordered pits in the walls of tracheids (Heady 2002). The annual growth rings visible in Wollemi Pine wood make it a candidate for dendrochronological study. However, the Wollemi Pine also exhibits strongly asymmetric radial growth, which means that rings may not be visible on all axes every year (Baker & Simkin 2010), leading to a ‘wedged’ appearance of rings when the trunk is viewed as a cross section. This means that the best samples for dendrochronological analysis are complete discs taken from fallen Wollemi Pine trunks, rather than less invasive core samples, to ensure a complete tree ring record. The longest chronologies recorded are in the order of 500 years (from an 18m trunk killed in the 2019-20 fires; Mackenzie et al. unpubl. data) and 400-450 years (from the widest known fallen trunk in the wild with a DBH of c. 90 cm; Banks 2002; Backer & Simkin 2010). A second challenge is using tree rings to estimate tree age as it is likely that stems or trunks originating from seed would have grown for many years before reaching the height at which the sample was taken (often at breast height, 1.35 m for example) although coppice stems on established plants can reach this height within a shorter time (Mackenzie et al. unpubl. data); hence, age estimates of trunks should be considered as minimum estimates only (Baker & Simkin 2010). In the case of coppice trunks on larger individuals, there is no way of knowing how old the individual tree (the genet or subtending rootstock) was before it produced a particular trunk (ramet) and therefore it is impossible to age an individual tree (this is not the case for smaller saplings originating from seed). Nevertheless, the abovementioned chronologies indicate that individual trunks can reach over 500 years old (Banks 2002; Baker & Simkin 2010; Mackenzie et al. unpubl. data).
The Wollemi Pine’s exceptionally low genetic diversity (Peakall et al. 2003), reported in the previous recovery plan (NSW DEC 2006a), was confirmed by Greenfield et al. (2016). The Wollemi Pine chloroplast genome has also since been sequenced (Yap et al. 2015). More refined population genetic studies based on reduced representation genome sequencing approaches are currently in progress. Preliminary analyses have detected low-level variation within and between stands (Bragg et al. unpubl. data) and the genome of the Wollemi Pine has recently been mapped (Stevenson et al. 2023 preprint). Analysis of the Wollemi Pine genome indicates that its population expanded rapidly 8-6 million years BP, before shrinking to approximately 20% of its size 7-3 million years BP (Stevenson et al. 2023 preprint). Wollemi Pine has existed as a relictual population since 120,000 years BP (Australia’s last major glacial/drying period). Population genomic analysis revealed high levels of inbreeding and clonal propagation with extremely low heterozygosity, even for a gymnosperm genome (Stevenson et al. 2023 preprint). This low genetic diversity may contribute to low rates of seed viability in the Wollemi Pine. Implications for the Wollemi Pine’s ability to adapt to changing conditions are the subject of ongoing research; this is also likely to have implications for the adaptability, or lack thereof, of the species to respond to invasive pathogens and pests.
The Wollemi Pine is restricted to specialised habitats in rainforest communities in deep sandstone gorges, which maintains a closed canopy vegetation structure with an understorey of shrubs or juvenile trees, and a dense layer of ferns. Disturbance events in this community are important for producing canopy gaps that may be necessary for successful regeneration in Wollemi Pine.
Fire
The summer of 2019–20 was the first opportunity since the discovery of the Wollemi Pine to study the response of wild individuals to fire (Mackenzie et al. 2021). Fire scars on the trunks of most mature adults at the time of the species’ discovery indicate that the groves have been impacted by, and survived, past wildfires (NSW DEC 2006a). The coppicing habit of the Wollemi Pine may have helped it survive previous fires as it allows for the development of new shoots to replace fire-killed trunks (Burrows et al. 2003).
Field surveys of the wild Wollemi Pine population after the 2019–20 bushfires revealed that most adult trees survived the fires with their upper canopies intact (Mackenzie et al. 2021). Of the fire-affected trees greater than or equal to 2m tall, two-thirds suffered partial canopy loss and one-third (predominantly juvenile trees) were completely top-killed (Mackenzie et al. unpubl. data). Of these top-killed individuals, approximately one-third were killed outright with the remainder (c. 20% of the post-fire population) reduced to vulnerable basal resprouts (Mackenzie et al. unpubl. data). The pre-fire bank of seedlings and smaller juveniles < 2m tall has been 92–95% eliminated with only 4–7% of pre-fire individuals successfully resprouting (Mackenzie et al. unpubl. data). However, this bank of smaller juveniles is expected to re-establish from seed over the next 20-30 years (Mackenzie et al. 2021).
Drought
Wollemi Pine is susceptible to the impacts of drought, exhibiting crown-death when under water-stress and tissue damage when exposed to high temperatures (Offord 2011; Zimmer et al. 2015a). However, the steep-sided sandstone gorges which the Wollemi Pine occupies provides some refuge from the impacts of drought (NSW DEC 2006a). Although drought events may contribute to canopy gap formation via crown-loss and mortality in competing canopy species, it is likely that any significant drought event which impacts the Wollemi Pine’s habitat would result in declines in population health and size due to the species sensitivity to these events.
Windstorms
Banks (2002) noted a “horseshoe-shaped flaw” in the wood of a fallen Wollemi Pine trunk from the wild population, which he suggests was similar to flaws in Araucaria cunninghamii (hoop pine) wood that have been attributed to wind storms (“which bend trees until the bole elasticity is exceeded, causing internal failures”). The shattered base of the fallen Wollemi Pine trunk in question provided further evidence for the impact of severe wind on the wild stands (Banks 2002). Whether emergent species such as the Wollemi Pine are generally more likely to be damaged by wind has not yet been resolved, as damage can be influenced by multiple and interacting factors including stem size, species, stand conditions and plant health, not to mention abiotic factors including topography, disturbance history and soil (Everham and Brokaw 1996).
Rock and tree falls
Little is known about the influence of rockfall and surface instability on the population dynamics of the Wollemi Pine, but it is thought to be an important factor. The sandstone on which Wollemi Pine occurs is known to be unstable. Indeed, rockfalls have been observed in situ. Rockfalls may cause mortality and injury to different plant species at different rates, mediating competitive interaction and influencing community composition. Injury through rockfall may also trigger coppicing. That Wollemi Pine exists on steep slopes may indicate it is more tolerant of, or better able to withstand, substrate instability than co-occurring species (such as, via physical protection from bark, or differences in root deployment and penetration into the sandstone). Tree and rock falls damaged several Wollemi Pine individuals during the 2019–20 fires, and the incidence of such disturbances has increased since the fires.

The Wollemi Pine is listed as Critically Endangered because of its very small population size and very limited geographic distribution – these characteristics mean that it is at an increased risk of extinction due to catastrophic events, such as bushfires or disease outbreaks. The wild Wollemi Pine population is also threatened by disturbance via site visitation, both from authorised essential management and research as well as unauthorised visitation resulting in trampling, soil compaction or erosion and spread of pathogens. This disturbance can impact habitat stability, recruitment and cause dieback and mortality, although numerous strict regulations currently govern access to the wild population and mitigate unauthorised visitation. Other threats include dieback caused by Phytophthora infection, fire regimes that cause declines in biodiversity (especially high frequency fire), pest and weed incursion, and loss or restriction of habitat due to anthropogenic climate change.
Small population and restricted geographic distribution
The wild Wollemi Pine population consists of just 45 mature individuals and 46 juveniles (Table 1) which occur over an area of only 4 km2. These characteristics increase the species risk of extinction due to a catastrophic event, such as a severe bushfire, drought or disease outbreak.
The bimodal size structure of the wild Wollemi Pine population is indicative of an apparent recruitment bottleneck that remains a serious concern for the population’s long-term viability. Zimmer et al. (2014) suggested that the infrequency of successful transitions between juvenile and adult Wollemi Pine is typical of many rainforest species that maintain banks of seedlings and small juveniles that respond to stochastic increases in resource availability (often via canopy gap formation; Whitmore 1989). However, subsequent research suggests that this recruitment bottleneck is fire-related (Mackenzie et al. unpubl. data). The near-complete loss of the seedling and small juvenile bank in the 2019–20 bushfires is a significant concern. Re-establishment of this juvenile bank from seed will require a fire-free period of at least 20–30 years (Mackenzie et al. 2021), and many more decades without fire for replacement of larger individuals, which may be unlikely without management intervention due to increasing trends in fire risk along the east coast (Dowdy et al. 2015).
Unauthorised visits to the wild Wollemi Pine population are illegal and have the potential to damage Wollemi Pine individuals (especially seedlings and small juveniles which are very difficult to discern in the understorey), trample and erode fragile soils, and result in further introduction and spread of pathogens that may affect the health and viability of the population. Additionally, following its discovery, the species was at a high risk of damage from illegal collection by unauthorised visitors; however, this risk has declined significantly following the successful cultivation and commercialisation of Wollemi Pine cuttings.
Knowledge of the location of the wild population is restricted to the minimum essential staff. All visits to the population by NSW DCCEEW and NPWS employees, or by other approved persons, are conducted in accordance with the ‘Wollemi Pine Access Strategy’ developed by the Wollemi Pine Recovery Team and NPWS. The strategy details policies and procedures that include site hygiene protocols and within-stand access protocols to mitigate the risk of pathogen introduction or spread, and limit site access to activities critical to the conservation of the species and its habitat. The strategy also includes a requirement for NPWS to maintain a register of all personnel visiting the stands and the purpose of the visits.
Site surveillance is conducted to discourage and detect illegal, unauthorised visitation to the stands, and any breaches of notified closures under the National Parks and Wildlife Regulation 2019 (NPW Regulation) are investigated by NPWS. The known stands of Wollemi Pine are protected under both state and federal legislation, including through the declared AIS and AOBV, which provide legal protection for the Wollemi Pine and its habitat from damage and unauthorised visitation. Further legal protections for the Wollemi Pine are provided via the restriction of access through the AOBV declaration and associated clauses in the BC Regulation.
Infection by pathogenic Phytophthora species
Infection with pathogenic Phytophthora species is generally understood to be one of the greatest threats to the Wollemi Pine. Phytophthora is a genus of water mould, spread primarily by contaminated soil, plant material or water and commonly infecting the roots of susceptible plants, causing dieback and death (DoEE 2018). Since initial research showed severe impacts of P. cinnamomi on seedlings under laboratory conditions (Bullock et al. 2000) and the discovery of P. cinnamomi and P. multivora in Stand 1 in 2005 (Salleh 2005) and 2015 (Puno et al. 2015), respectively, significant effort has been invested in preventing the introduction and spread of soil-borne pathogens within and between the wild stands (such as, through the use of the site hygiene policy), and in the treatment of P. cinnamomi at Stand 1. The effects of infection by Phytophthora spp. can vary through time, and among individuals and may vary with climate, as additional stressors may increase the effects of the infection (Davison 2011).
Research has been undertaken on cultivated Wollemi Pine plants to develop treatment methods to mitigate the impacts of pathogenic Phytophthora. Laboratory and greenhouse experiments have found that painting phosphonate onto the bark of mature Wollemi Pines can reduce the impacts of P. cinnamomi (Liew & Phelan, unpubl. data). While these results are promising, they have important limitations: primarily that the experimental work was conducted on only 20 trees, and that these trees, while the largest available in the ex situ collection, were only 22–24 years old. As such they were relatively young and small compared with most trees in the wild population. Results showed that the technique is effective and provides protection to Wollemi Pine up to 55 weeks or more (E. Liew, pers. comm). However, this estimate could be refined for Wollemi Pine of different sizes by further testing.
Since the previous recovery plan was published, another species of pathogenic Phytophthora has been detected in Stand 1: P. multivora (Puno et al. 2015). No pathogenic Phytophthora species have been detected in soil samples from the other stands (E. Liew, pers. comm.); however, new diagnostic developments which allow better detection and differentiation of Phytophthora species are yet to be used on soil samples taken from the wild stands (B. Summerell, pers. comm.).
Fire regimes that cause declines in biodiversity
While the Wollemi Pine has some capacity to resprout after low severity fires, this capacity varies markedly between life history stages (Mackenzie et al. unpubl. data) and the consequences of high severity fire are likely to be catastrophic. The species (especially smaller individuals) is vulnerable to high frequency fire (Mackenzie & Auld in press; Mackenzie et al. unpubl. data). Repeated short-interval fires will lead to increased rates of mortality due to reductions in protective tissues around meristems and reduced rates of resprouting due to reductions in availability of photosynthates for resprouting. Short fire intervals also mean less time for seedlings and juveniles to develop fire resistance and grow to heights beyond which their canopies may survive fire impacts (Mackenzie et al. unpubl. data).
The risk of unplanned fire in the wild population was substantially (albeit temporarily) reduced as a result of the 2019–20 fires which consumed surface fuels that had accumulated on site. This risk was expected to remain low for approximately five years (until 2024) until significant re-accumulation of surface fuels (Mackenzie et al., 2021). Beyond this, long-term fire exclusion in the order of 50–100 years is required to facilitate population recovery and persistence (Mackenzie et al. 2021).
The ongoing risk of fire is managed by NPWS, and the declaration of 5,000 hectares of the Wollemi Pine catchment as an AIS triggers the integration of spatial information into NPWS fire management systems. This facilitates the rapid identification and protection of environmental and cultural assets (such as the Wollemi Pine) during planned and unplanned fires.
Pest and weed incursion
The 2019–20 bushfires were followed by three years of above average rainfall which resulted in a significant increase in feral herbivore populations and range expansions for feral pigs and deer. These conditions also resulted in significant growth of perennial weeds within the Wollemi Pine catchment area, including blackberry (Rubus fruticosus agg.), tree of heaven (Ailanthus altissima) and Madeira vine (Anredera cordifolia). While restricted populations of a range of pests and weeds existed within the catchment before 2019, expansion of pest and weed populations following ideal growing conditions requires significantly more effort to manage potential risks.
Previous recovery plans did not identify pest incursion as a threat to the Wollemi Pine. Additionally, changing climate and increased storm severity (per climate change projections) has seen stream bank damage and disturbance that provides ideal growing conditions for a range of weed species that may not have been a threat until recently.
Feral deer and pigs, while not yet detected in the wild stands, are present in the immediate catchment area. These species are vectors for weeds and soil pathogens, and funded control is essential to ensuring populations do not increase significantly and directly impact the Wollemi Pine.
Climate change including drought and temperature extremes
Understanding the physiological limits of the Wollemi Pine is particularly important in the context of climate change, where extreme weather events (such as heat waves and droughts) are predicted to occur more frequently and with increasing intensity (Seneviratne et al. 2012; Ukkola et al. 2020).
Wollemi Pine is known to incur tissue damage at and above 37.5 °C, which is the lowest temperature for damage initiation among its Australian Araucariaceae relatives (Offord 2011). By contrast, the Wollemi Pine has the greatest tolerance for cold, with damage only beginning at -6.8 °C (Offord 2011), and cultivated Wollemi Pines growing in cooler regions, such as at BMBGMT, appear to suffer less heat-stress related damage and mortality than those growing in hotter regions, such as at ABGMA (C. Offord, pers. obs cited in Offord & Zimmer 2023; see Offord & Zimmer 2023 for climate profiles of these locations). Additionally, the response of Wollemi Pine to drought in laboratory experiments was noted as remarkably different to other Australian species within the Araucariaceae (Zimmer et al. 2015b). These other Australian Araucariaceae species were isohydric, meaning they close stomata in response to water stress, ceasing gas exchange and photosynthesis, with the aim of maintaining leaf water potential and avoiding damage. The Wollemi Pine, by contrast, continued to photosynthesise as water stress increased, leading to declining leaf water potentials, and eventually damage—manifested in crown mortality (both stem and branch mortality) (Zimmer et al. 2015b). A contributing factor to the inability of the Wollemi Pine to avoid damage may be the vasoconstriction at its branch bases, as described by Burrows et al. (2007). However, the species is also able to resprout after drought-induced damage, providing some post-drought recovery potential. Furthermore, Lewis et al. (2015) examined the interactive effects of increased CO2 and temperature on Wollemi Pine growth and physiology in glasshouse conditions. While elevated CO2 increased plant growth, they concluded that increasing temperatures may offset these effects by affecting respiration rates.
This research, and the Wollemi Pine’s restricted distribution in a refugia habitat, indicates that the species is vulnerable to the impacts of temperature extremes and drought. Anthropogenic climate change is projected to result in increases in average temperatures, the severity and length of fire seasons and the intensity of extreme weather events, and a decrease in cool season rainfall across much of eastern Australia (CSIRO & Bureau of Meteorology 2022). These changes, which are likely to include a substantial increase in the peak temperature on hot days along the east coast (Dowdy et al. 2015), could severely impact the Wollemi Pine and its habitat. In combination with the increased risk of extreme fire seasons (Canadell et al. 2021; van Oldenborgh et al. 2021), this highlights the need for greater understanding of the nature of climate change impacts on the wild Wollemi Pine population.

By 2050, the wild Wollemi Pine population will exist as a resilient network of stands throughout numerous gully locations within Wollemi National Park, including translocated stands that are on track to become self-sustaining and which occur in habitats that are resilient to the impacts of a changing climate. Within all stands, the threats to the species will be understood and well managed to the extent practicable. Additionally, the full range of genetic diversity in the wild population will be represented and backed up in multiple national and international ex situ living collections and supplemented by genetically diverse seed accessions.
The objectives of this recovery plan are that by 2034:
the wild Wollemi Pine population and its habitat are protected from threatening processes to the extent practicable, and have not decreased in size or quality
understanding of the biology of the Wollemi Pine and co-occurring species in its natural habitat has been improved and informs the conservation of the species and its habitat
representative ex situ collections are established and maintained
translocated stands of Wollemi Pine are maintained, and additional translocations are undertaken, as required
community and Indigenous awareness of the Wollemi Pine and engagement with recovery efforts is maintained and improved.
The actions listed under each recovery objective below detail the activities required to achieve recovery of the Wollemi Pine. The performance criteria will be used to evaluate the degree to which each objective has been successfully achieved.
Performance criteria for recovery objective 1:
By 2034, the size of the wild Wollemi Pine population has been maintained or increased compared to current population estimates with no loss of genetic diversity and no declines in plant health.
By 2034, the extent of pathogens such as Phytophthora spp. in the wild stands is being controlled, further infections are being halted, and infected trees are being effectively treated to the extent practicable.
By 2034, the risk of damage to the wild Wollemi Pine stands from bushfire and bushfire management activities has been reduced.
By 2034, the wild Wollemi Pine population remains free of invasive woody weeds, vertebrate pest impacts, and adverse changes in water quality and hydrology.
Table 2 Actions to protect and maintain the wild population and its habitat
Action | Description | Detail |
1.1 | Protect the wild population from unauthorised visitation by continuing to implement the Wollemi Pine Access Strategy. | The Wollemi Pine Access Strategy aims to minimise damage to Wollemi Pines through trampling, soil compaction or erosion, spread of pathogens, which impact habitat stability, recruitment and cause dieback and mortality. The Strategy describes the circumstances in which authorised personnel may lawfully visit the Wollemi Pine wild population to conduct essential research, monitoring or management activities. |
1.2 | Develop and implement a disease management plan to reduce the spread and impact of pathogens, particularly Phytophthora cinnamomi and P. multivora, on the wild Wollemi Pine population. | To protect the Wollemi Pine from the impacts of pathogenic Phytophthora species and other microbial pathogens, it is necessary to prevent the introduction of novel pathogens and the spread of existing pathogens within stands. This is to be achieved by the development and implementation of a disease management plan and risk mitigation protocols developed in line with the requirements of the CAP. These documents will be utilised in conjunction with pre-existing standard operating procedures for hygiene management and the Wollemi Pine Access Strategy to protect the species from the spread of pathogens. The following guidelines are to be used in development of the management plan and risk mitigation protocols. Prevention: - Restrict visits to the wild stands as per Action 1.1.
- All authorised personnel visiting the wild Wollemi Pine population will be required to thoroughly disinfect all footwear, clothing and equipment in accordance with the adopted Site Hygiene Protocol prior to entering the area.
- Reduce populations of disease vectors as per Action 1.5.
Control/mitigate impacts: - Conduct soil sampling and analysis in response to observations of symptomatic Wollemi Pine or co-occurring species.
- Any further outbreaks of pathogens in the stands will be treated as an emergency and dealt with promptly and effectively using expert advice available through the RBGDT, NSW Department of Primary Industries and the national Phytophthora Threat Abatement Plan working group.
- Where practical, symptomatic trees will be treated according to the disease management plan.
Research, consultation and improvement: - Research outcomes will inform future treatment methods as per Action 2.5.
- In accordance with the CAP, the Recovery Team will regularly review approved procedures for minimising the risk of introducing pathogens to the wild Wollemi Pine population, and to translocated stands, as appropriate.
- The Recovery Team will maintain a list of appropriate contacts within NSW DCCEEW, RBGDT and other organisations with Phytophthora and other pathogen management expertise.
|
1.3 | Develop and implement the Wollemi Pine Fire Management Strategy. | The Wollemi Pine Fire Management Strategy, which is being developed under the CAP, provides additional actions to deliver tailored wildfire suppression critical to the conservation of the Wollemi Pine and its habitat. The strategy details advanced fire suppression actions including broadscale catchment hazard reduction activities and continuation of rapid aerial response programs for early detection and suppression of wildfires, many of which are already being implemented. Data logging of site conditions of the wild population will be used to inform appropriate responses to drying conditions and increasing fire danger. As this technology continues to advance this may be able to occur remotely. |
1.4 | Manage weeds in the Wollemi Pine catchment. | Annual monitoring of blackberry and other woody perennial weed incursions in the catchment of the wild Wollemi Pine population will be carried out and treatment of infestations will occur when NPWS, in consultation with the Recovery Team, concludes that the infestation could adversely affect the wild Wollemi Pine population. Weed control within the Wollemi Pine catchment will be carried out using best practice and low-impact methods. |
1.5 | Manage pest species in the Wollemi Pine catchment. | Pest management operations are undertaken for feral pig (Sus scrofa), feral goats (Capra hircus), fallow deer (Dama dama) and red deer (Cervus elaphus). Aerial shooting of both deer and pigs has been undertaken annually and numbers culled between 2019 and 2023 have significantly increased, indicating that populations have also significantly increased. NPWS will continue to work with neighbours and Local Land Services to prioritise and coordinate pest animal control within the Wollemi Pine catchment and identify additional pest species. |
1.6 | Manage the risk of adverse changes in water quality and hydrology in the Wollemi Pine catchment. | NPWS will seek the co-operation of all relevant authorities and stakeholders, if appropriate, in the management of water quality and hydrology in the catchment. Through this action, NPWS will ensure that the risk of pollution (including chemical, oil and fuel spills) and other adverse changes to water quality and the hydrology of the catchment of the Wollemi Pine stands are minimised. |
1.7 | Conduct further systematic surveys to detect previously unknown stands of Wollemi Pine. | Surveys will be conducted throughout WNP and surrounds to detect any undiscovered Wollemi Pine stands and facilitate their active management and conservation. Where applicable, new technologies and advanced survey techniques will be used to identify suitable locations to survey. |

Recovery objective 2: Understanding of the biology of the Wollemi Pine and co-occurring species in its natural habitat has been improved and informs the conservation of the species and its habitat.
Performance criteria for recovery objective 2:
By 2034, the Wollemi Pine monitoring and research programs yield rigorous data that support effective in situ management via:
i. increased understanding of Wollemi Pine ecology and population dynamics, including population genetics; recruitment dynamics of seedlings (new genets) and of stems (new ramets) on juveniles and adults; and the impact of fire on the species and its habitat
ii. early detection of declines in plant health and loss of stems (ramets) or entire individuals (genets), thereby maximising opportunities for intervention, including disease treatment
iii. evaluation of the efficacy of management interventions.
Table 3 Actions to improve understanding of Wollemi Pine biology and ecology
Action | Description | Detail |
2.1 | Continue to undertake monitoring that will assist with the management and conservation of the species and its habitat. | The aims of the current monitoring strategy are fourfold: - Population structure: census the number of established adults, juveniles, and seedlings every 5 years, and after any fire or other significant disturbance event affecting the population.
- Stem health: annually assess the proportion of individual genets and ramets showing signs of disease at their bases.
- Canopy health: annually assess canopy health to identify canopy dieback, disease and disturbance impacts through high resolution photography and drone imagery.
- Recruitment: count, measure and map seedlings and vegetative recruits (coppices ≥ 2 m tall and/or ≥ 2 cm DBH every 5 years.
- Site conditions: monitor wild site conditions, including temperature, rainfall and fuel loads.
Any additional in situ monitoring and research programs will be subject to review and approval by the Wollemi Pine Recovery Team. |
2.2 | Conduct research to improve understanding of the population dynamics of the Wollemi Pine, including the species’ persistence and regeneration niches, its response to fire, the dynamics of the rainforest community, the impacts of climate change and the drivers of cone production. | There have been significant advances in our understanding of Wollemi Pine ecology since the species’ discovery, however, some important aspects require further research. These include: - Characterising the persistence and recruitment niches, respectively, of Wollemi Pine.
- The dynamics of the rainforest community inhabited by Wollemi Pine, including functional relationships between Wollemi Pine and other key rainforest species that are relevant for habitat management, such as how the 2019–20 bushfires have impacted canopy cover, the amount of light reaching the understorey and its influence on growth and survival of seedlings and juveniles.
- How different life history stages of Wollemi Pine respond in situ to fires of varying severity, frequency and seasonality, and how these responses vary between wild and translocated individuals (and, in the case of the latter, seed- versus cutting-grown plants), thereby improving our knowledge of the fire regimes that Wollemi Pine can tolerate and improving fire management strategies for conservation of the wild population and translocated stands.
- Other fire-related research questions, including:
- How can the risk of future wildfires impacting the wild stands be predicted effectively (what are the key drivers of fire risk in that habitat, including the impact of a changing climate on fire risk)?
- What are the most appropriate, effective, and ecologically sustainable measures to suppress fires approaching the wild stands and to mitigate fire impacts within the stands (such as, irrigation, manual fuel removal, wrapping vulnerable trunks with fire-retardant material)?
- What are the effects of fire-retardant chemicals on Wollemi Pine and co-occurring species?
- What are the effects of fire suppression on co-occurring species?
- The long-term impacts of climate change on the Wollemi Pine and co-occurring species.
- Factors affecting the timing and quantity of cone production in Wollemi Pine.
- The impact of vertebrate and invertebrate herbivores on Wollemi Pine seedlings and small juveniles (exclosure study).
|
2.3 | Continue research on the genetic diversity of the wild Wollemi Pine population. | The species’ genome has recently been mapped (Stevenson et al. 2023 preprint). Ongoing research (Bragg et al. unpubl. data) aims to characterise the genetic variation among wild Wollemi Pine individuals. This research will further guide the development of ex situ collections (Actions 3.1 & 3.2) and translocated stands (Actions 4.1–4.5) to maximise their genetic diversity (Mackenzie et al. 2021). Research will aim to address the following knowledge gaps: - genetic variation and gene flow within and between Wollemi Pine stands
- delineating distinct genetic individuals within the larger stem clusters
- extent of cross-pollination versus selfing
- morphological and other character variations.
|
2.4 | Undertake further research into treatment methods to mitigate the impacts of pathogenic Phytophthora. | Ex situ research has been undertaken on Wollemi Pine to develop treatment methods to mitigate the impacts of pathogenic Phytophthora. Early work highlights the potential for phosphonate bark painting to reduce the impacts of P. cinnamomi. The next stages of this work include: - Development of a disease management plan, including appropriate triggers for field application of phosphonate and/or other treatments to wild Wollemi Pine (as per Action 1.2).
- Monitoring the response of field-treated individuals.
- Utilising research outcomes to inform the development of a disease management plan as per Action 1.2.
- Refining the duration of protection for Wollemi Pine of different sizes/ages, thereby facilitating optimal timing of booster applications.
- Continue to investigate the effectiveness of alternative treatment methods.
|
Recovery objective 3: Representative ex situ collections of Wollemi Pine are established and maintained.
Performance criteria for recovery objective 3:
By 2034, representation of extant wild individuals in the main ex situ potted collection at ABGMA is increased towards 100% with a minimum of three replicate clones per genotype; the diversity of maternal lines represented in the seed bank is increased; and the collection is being maintained in line with ex situ collection risk management requirements.
By 2034, a comprehensive in-ground backup of the ABGMA living collection has been established at BMBGMT and the collection is being maintained in line with ex situ collection risk management requirements.
By 2034, resilience of the ABGMA and BMBGMT living collection and seed accessions to climate change has been achieved through the establishment of additional national and international backup collections, as required.
Table 4 Actions to establish and maintain ex situ collections
Action | Description | Detail |
3.1 | Maintain a representative seed bank and off-site living collection of the wild population at ABGMA | The further development and maintenance of representative off-site collections is considered critical for the long-term conservation of the Wollemi Pine, in case of severe decline of the wild population, and to provide material for translocation, research and education. Cuttings of all putative individuals in the wild population will be collected as soon as practicable after they are identified, followed by propagation and addition to the main living collection at ABGMA. Ex situ collections are to be maintained in line with Australian best practice guidelines (Commander et al. 2018; Martyn et al. 2021) and the Botanic Gardens of Sydney Living Collections Strategy (BGoS 2023). Wild individuals containing significant new genetic variation that is not currently represented in the ex situ collection should be prioritised for collection and propagation. Exceptions will be made where collection places undue risk to individual Wollemi Pines or staff safety. Cutting collection and propagation priorities will be informed by the results of ongoing population genomics research. Increasing the diversity of maternal lines represented in the ABGMA seed bank and backing up this collection is a high priority. Sourcing seeds from genetically diverse collections of cultivated plants, such as mature Wollemi Pines at BMBGMT, should be prioritised over collecting seeds from the wild population of Wollemi Pine wherever possible. |
3.2 | Establish comprehensive backups of the ABGMA seed bank and living collection | BMBGMT provides the main local backup of the ABGMA living collection in a contemporaneously favourable climate, as well as providing the most genetically diverse source of seeds with known material lines currently available for seed banking, propagation and research. Priority actions include: - Increasing representation in the BMBGMT living collection towards 100% of known extant genotypes in the wild population (such as via conservation hedges and/or growing trees to maturity to provide additional sources of seeds).
- Ongoing seed collection from mature Wollemi Pine at BMBGMT and banking seed accessions at ABGMA, including developing sustainable methods of seed harvesting (such as via seed baskets and/or the use of drones).
- Establishing representative backup seed accessions at other national and international institutions.
Additional national living collections at higher (southern) latitudes (such as Vic or Tas) are also desirable to provide climate change resilience while enabling relatively easy repatriation of material to NSW, if required. Priority actions in this respect include identifying appropriate locations and partner organisations, curation arrangements, and the support required. While international collections such as the BGCI metacollection provide the greatest resilience to climate change and other stochastic disturbances potentially impacting the ABGMA and BMBGMT living collections, repatriation of material to NSW is expected to be difficult due to Australian biosecurity and quarantine restrictions; hence, such collections are considered a lower conservation priority. |
3.3 | Conduct research to support the ex situ conservation of Wollemi Pine, including growth in cultivation and seed storage. | Recent increases in the availability of cones, seeds and seedlings have created new research opportunities, including: - Investigating the advantages and disadvantages of using clones vs seed-grown plants in maintaining and maximising the genetic diversity of ex situ collections and in translocations.
- Optimal methods for long-term storage of Wollemi Pine seed.
- Improving understanding of the species breeding system.
Priorities for future studies involving seeds and cultivated plants are: - How to maximise the storage capacity in conventional seed banks at -18 °C and/or develop alternative seed banking techniques (such as higher temperature but sub-zero storage such as -5 to -10°C).
- The feasibility of storing seeds or embryonic axes cryogenically (in liquid nitrogen at less than -180°C).
- Environmental and genetic factors involved in seed viability. This includes understanding whether this species has ‘mast’ events.
- Understanding the embryological processes in seed production.
- Management of seed production in cultivation (maximising seed production, genetic diversity and viability).
- Capitalising on the opportunities presented by the recent metacollection distribution.
- Working with the International Conifer Conservation Programme and partner gardens to monitor plants in the international metacollection to understand environment adaptability and growth factors in cultivation under different climates, and to guide future translocation and assisted migration efforts.
|
Recovery objective 4: Translocated stands of Wollemi Pine are maintained and additional translocations are undertaken, as required
Performance criteria for recovery objective 4:
By 2034, established translocated stands are progressing towards becoming self-sustaining and contributing to increasing the size and geographic distribution of the wild Wollemi Pine population. Indicators of success include ≥ 50% survival and sustained growth of translocated saplings, and, potentially, maturation of at least some individuals.
By 2034, options for further increasing the size and geographic distribution of the wild Wollemi Pine population and protecting it against climate change impacts using translocation have been evaluated and progress is made towards their implementation, where appropriate.
Table 5 Actions to establish and maintain translocated stands
Action | Description | Detail |
4.1 | Continue management and monitoring of the pilot translocation site at BMBGMT. | Key ongoing tasks are to: - Continue annual monitoring.
- Understand the impacts of the 2019–20 fires on plant survival.
- Maintain the site hygiene protocol and revise where appropriate to prevent infection with pathogenic Phytophthora species.
- Maintain the access track.
- Include the translocation site in BMBGMT site management plans, including for public access, weed control and fire.
- Investigate opportunities for continued research and education at the pilot translocation site.
|
4.2 | Continue monitoring of T1 and T2 translocated stands in Wollemi National Park and conduct further augmentations using adaptive management. | Key ongoing tasks are to: - Continue annual monitoring (number of established juvenile and adult individuals, height and number of branches per plant, canopy health, cone production, and seedling recruitment).
- Continue population augmentation as per the 2019 translocation plan.
- Implement the site hygiene protocol and site access policy to minimise impacts (such as trampling of plants, compaction and erosion of soils, and the introduction and spread of pathogens and weeds).
- Quantify the impacts of any disturbances (such as wildfire, drought, flood) on the translocated stands.
|
4.3 | Evaluate and plan for further translocations, as required | Progress towards a self-sustaining wild population of the Wollemi Pine, increasing its geographic distribution and total population size, and increasing its resilience to the impacts of climate change (including fire and drought) are key goals of this recovery plan. Due to the species’ limited dispersal capacity and the current recruitment bottleneck, key mechanisms to achieve these goals include, conducting augmentation of the wild population and/or existing translocated stands and/or establishing additional translocated stands within and beyond WNP. However, translocation (including augmentation, reintroduction and introduction) can be complex procedures which pose numerous ecological and philosophical challenges and considered planning and consultation are required to conduct these actions appropriately. An ongoing translocation review process will be implemented to identify and evaluate translocation options, update guidelines, and prepare and consult on translocation plans, as required. As part of this translocation review process, efforts will be made to identify potential translocation sites which are likely to remain suitable under various climate scenarios. |
4.4 | Prepare material for translocation, as required. | Custom-produce healthy, acclimatised plants of known genetic provenance for any additional translocations, as required. Given stewardship of the main ex situ collection and the associated staff expertise, ABGMA is the best source of plant material for translocation. |
4.5 | Undertake further translocations, as required | Augment the existing wild population and/or translocated stands, and/or establish additional translocated stands, especially in habitat resilient to climate change. Any translocation work should be conducted in accordance with the translocation review process (Action 4.3) and established translocation guidelines. |
Recovery objective 5: Community and Indigenous awareness of the Wollemi Pine and engagement with recovery efforts is maintained and improved
Performance criteria for recovery objective 5:
By 2034, community appreciation of and support for the conservation and protection of the Wollemi Pine is enhanced.
By 2034, a representative recovery team continues to coordinate and report on the implementation of the recovery plan and its effectiveness.
Table 6 Actions to improve community and Indigenous engagement with recovery efforts
Action | Description | Detail |
5.1 | Develop and maintain relationships with First Nations to facilitate consultation and allow free prior informed consent to be obtained regarding Wollemi Pine land management matters, where appropriate. | NPWS are working with Aboriginal groups across NSW to develop a range of joint management and partnership agreements. This will help ensure the continuing practice of traditional and contemporary culture in the management of the land, foster stronger relationships between the NPWS and Aboriginal communities, and provide benefits to the parks system and broader community of NSW. NPWS will work to include, where appropriate, Wollemi Pine land management matters in these ongoing consultation processes. Additionally, NPWS will continue to maintain relationships with Aboriginal interest groups and consult, where appropriate, on Wollemi Pine land management matters that impact Aboriginal natural and cultural heritage values. |
5.2 | Identify opportunities for community and First Nations involvement in Wollemi Pine conservation activities in ex situ locations. | Existing ex situ conservation projects for the Wollemi Pine, such as cultivation in botanic gardens, will be reviewed to identify opportunities for engagement with community and First Nations. |
5.3 | Develop educational displays at botanic gardens, targeted interactions with media, and advocates for dispersal of conservation messaging across social media and other platforms | Numerous articles (including peer-reviewed scientific articles, magazine and newspaper articles), webpages, TV and radio interviews, and educational materials (brochures, posters and videos) have been produced since the discovery of the Wollemi Pine in 1994. Many of these materials are summarised and cited in this recovery plan, while others can be found through internet searches. Priorities for ongoing communication include: - The importance of not attempting to locate or visit the wild stands;
- A focus on the ex situ collection of Wollemi Pines, highlighting that people can see and enjoy Wollemi Pines in botanic gardens or grow their own;
- The risk posed by pathogenic Phytophthora species and other pathogens, and the phytosanitary measures required to prevent the spread of Phytophthora (as per the Phytophthora TAP) and other diseases; and
- Leveraging the status of the Wollemi Pine in media interactions to raise the profile of other threatened species and ongoing efforts to conserve them.
|
5.4 | Maintain an effective and representative recovery team to oversee coordination and implementation of the recovery plan and coordinate reporting. | An effective and representative governance model is critical to coordinate implementation of actions, facilitate collaboration between partners, adaptively manage the recovery program, raise awareness, and communicate implementation progress through regular reporting. Governance arrangements should be agreed to by recovery team members and recorded for reference. |
The Wollemi Pine Recovery Team will be the key governance mechanism to coordinate recovery effort; provide advice; ensure appropriate progress in, and reporting on implementation; share and review information; and identify funding opportunities. Table 7 provides estimated costs, timelines and priorities for each of the Actions in the recovery plan for the first five years of its implementation. The Wollemi Pine Recovery Team will be responsible for reviewing and updating these costs, timelines and priorities as necessary.
Prioritisation
While all recovery actions are deemed necessary to halt decline and support recovery of the Wollemi Pine, priorities are assigned to actions in Table 7 to indicate the difference in their urgency. Definitions for priority ratings are:
Priority 1 (very high) – Action is critical to mitigate key threats and ensure the viability of the wild population.
Priority 2 (high) – Action will inform the management and recovery of the Wollemi Pine.
Priority 3 (moderate) – Action is desirable, enhances broader engagement and will contribute to long-term recovery.
Table 7 Indicative priorities, timings and costs ($k) for the implementation of recovery actions
Action | Detail | Priority | Lead agency | 2024/25 | 2025/26 | 2026/27 | 2027/28 | 2028/29 | Total |
1.1 | Protect the wild population from unauthorised visitation by continuing to implement the Wollemi Pine Access Strategy. | 1 | NPWS | 62.0 | 54.0 | 56.7 | 59.6 | 62.5 | 294.8 |
1.2 | Develop and implement a disease management plan to reduce the spread and impact of pathogens, particularly Phytophthora cinnamomi and P. multivora, on the wild Wollemi Pine population. | 1 | RBGDT | 8.4 | 8.8 | 9.3 | 9.7 | 10.2 | 46.4 |
1.3 | Develop and implement the Wollemi Pine Fire Management Strategy. | 1 | NPWS | 31.5 | 50.5 | 52.5 | 36.5 | 38.3 | 209.3 |
1.4 | Manage weeds in the Wollemi Pine catchment. | 1 | NPWS | 36.8 | 38.6 | 40.5 | 42.5 | 44.7 | 203.1 |
1.5 | Manage pest species in the Wollemi Pine catchment. | 1 | NPWS | 42.0 | 44.1 | 28.9 | 48.6 | 51.1 | 214.7 |
1.6 | Manage the risk of adverse changes in water quality and hydrology in the Wollemi Pine catchment. | 2 | NPWS | 5.3 | 5.5 | 5.8 | 6.1 | 6.4 | 29.1 |
1.7 | Conduct further systematic surveys to detect previously unknown stands of Wollemi Pine. | 2 | NSW DCCEEW SEID | 36.8 | 0.0 | 0.0 | 0.0 | 0.0 | 36.8 |
2.1 | Undertake monitoring that will assist with the management and conservation of the species and its habitat. | 1 | NSW DCCEEW SEID | 43.5 | 23.6 | 24.8 | 26.1 | 27.4 | 145.4 |
2.2 | Conduct research to improve understanding of the population dynamics of the Wollemi Pine, including the species’ persistence and regeneration niches, its response to fire, the dynamics of the rainforest community, the impacts of climate change and the drivers of cone production. | 1 | NSW DCCEEW SEID | 10.5 | 11.0 | 11.6 | 12.2 | 12.8 | 58.1 |
2.3 | Continue research on the genetic diversity of the wild Wollemi Pine population. | 1 | RBGDT | 20.0 | 21.0 | 22.1 | 23.2 | 24.3 | 110.6 |
2.4 | Undertake further research into treatment methods to mitigate the impacts of pathogenic Phytophthora. | 2 | RBGDT | In-kind | In-kind | In-kind | In-kind | In-kind | 0.0 |
3.1 | Maintain a representative seed bank and off-site living collection of the wild population at ABGMA. | 1 | RBGDT | 105.0 | 121.3 | 115.8 | 133.7 | 127.6 | 603.4 |
3.2 | Establish comprehensive backups of the ABGMA seed bank and living collection. | 1 | RBGDT | 15.8 | 0.0 | 17.4 | 0.0 | 19.1 | 52.3 |
3.3 | Conduct research to support the ex situ conservation of Wollemi Pine, including growth in cultivation and seed storage. | 2 | RBGDT | 30.0 | 31.5 | 44.1 | 46.3 | 48.6 | 200.5 |
4.1 | Continue management and monitoring of the pilot translocation site at BMBGMT. | 2 | RBGDT | 5.0 | 5.3 | 5.5 | 7.5 | 7.9 | 31.2 |
4.2 | Continue monitoring of T1 and T2 translocated stands in Wollemi National Park and conduct further augmentations using adaptive management. | 1 | NSW DCCEEW SEID | 31.5 | 33.1 | 34.7 | 36.5 | 38.3 | 174.1 |
4.3 | Evaluate and plan for further translocations, as required | 1 | NSW DCCEEW SEID | 0.0 | 11.6 | 0.0 | 0.0 | 0.0 | 11.6 |
4.4 | Prepare material for translocation, as required. | 1 | RBGDT | 0.0 | 0.0 | 21.5 | 22.6 | 23.7 | 67.8 |
4.5 | Undertake further translocations, as required | 1 | NSW DCCEEW SEID | 0.0 | 0.0 | 57.9 | 18.2 | 19.1 | 95.2 |
5.1 | Develop and maintain relationships with Aboriginal interest groups to facilitate consultation and allow free prior informed consent to be obtained regarding Wollemi Pine land management matters, where appropriate. | 3 | NPWS | 0.0 | 2.8 | 2.2 | 0.0 | 0.0 | 5.0 |
5.2 | Identify opportunities for community and Indigenous involvement in Wollemi Pine conservation activities in ex situ locations. | 3 | RBGDT | 0.0 | 5.3 | 5.5 | 0.0 | 0.0 | 10.8 |
5.3 | Develop educational displays at botanic gardens, targeted interactions with media, and advocates for dispersal of conservation messaging across social media and other platforms | 3 | NPWS/RBGDT | 5.3 | 11.0 | 0.0 | 0.0 | 0.0 | 16.3 |
5.4 | Maintain an effective and representative Recovery Team to oversee coordination and implementation of the recovery plan and coordinate reporting. | 1 | NPWS | 1.4 | 1.5 | 1.6 | 1.6 | 1.7 | 7.8 |
| | | Totals: | 490.5 | 480.5 | 558.4 | 530.9 | 563.7 | 2624.3 |
3.4 Evaluation of the recovery plan
Implementation of recovery actions described in the recovery plan will be carried out by the nominated lead agencies identified in Table 7, and the implementation of recovery actions will be monitored and reported on by the Wollemi Pine Recovery Team.

Baker P & Simkin R (2010) Preliminary report on dendrochronological studies on the Wollemi Pine. Monash University.
Banks J (2002) Wollemi Pine: tree find of the 20th century, In: Dargavel J, Gaughwin D & Libbis B (eds) Australia’s Ever-changing Forests V, Proceedings of the Fifth National Conference on Australian Forest History. Centre for Resource and Environmental Studies, ANU, Canberra. pp. 85–89.
Benson J & Allen C (2007) Vegetation associated with Wollemia nobilis (Araucariaceae). Cunninghamia 10, 255–261.
BGoS (Botanic Gardens of Sydney) (2023) Living Collections Strategy. Botanic Gardens of Sydney, NSW.
Brophy JJ, Goldsack RJ, Wu MZ, Fookes CJ & Forster PI (2000) The steam volatile oil of Wollemia nobilis and its comparison with other members of the Araucariaceae (Agathis and Araucaria). Biochemical Systematics & Ecology 28, 563–78.
Bullock S, Summerell BA & Gunn LV (2000) Pathogens of the Wollemi pine, Wollemia nobilis. Australasian Plant Pathology 29 (3), 211–214.
Burrows GE (1999) Wollemi pine (Wollemia nobilis, Araucariaceae) possesses the same unusual leaf axil anatomy as the other investigated members of the family. Australian Journal of Botany 47 (1), 61–68.
Burrows GE (2012) Elusive but not hypothetical: axillary meristems in Wollemia nobilis. Annals of botany 109 (1), 1–2.
Burrows GE & Bullock S (1999) Leaf anatomy of Wollemi pine (Wollemia nobilis, Araucariaceae). Australian Journal of Botany 47 (5), 795–806.
Burrows GE, Meagher PF & Heady RD (2007) An Anatomical Assessment of Branch Abscission and Branch-base Hydraulic Architecture in the Endangered Wollemia nobilis. Annals of Botany 99 (4), 609–623.
Burrows GE, Offord CA, Meagher PF & Ashton K (2003) Axillary meristems and the development of epicormic buds in Wollemi pine (Wollemia nobilis). Annals of Botany 92 (6), 835–844.
Canadell JG, Meyer CP, Cook GD, Dowdy A, Briggs PR, Knauer J, Pepler A & Haverd V (2021) Multi-decadal increase of forest burned area in Australia is linked to climate change. Nature Communications 12, 6921.
Chambers TC, Drinnan AN & McLoughlin S (1998) Some morphological features of Wollemi pine (Wollemia nobilis: Araucariaceae) and their comparison to Cretaceous plant fossils. International Journal of Plant Sciences 159 (1), 160–171.
Commander L, Coates DJ, Broadhurst L, Offord CA, Makinson RO & Matthes M (eds) (2018) Guidelines for the translocation of threatened plants in Australia. Third Edition. Australian Network for Plant Conservation, Canberra.
Commonwealth of Australia (2024) Australia’s Strategy for Nature 2024-30. Commonwealth of Australia, Canberra.
Commonwealth of Australia (2020) Royal Commission into Natural Disaster Arrangements, Report. Accessed 19 May 2023.
CSIRO & Bureau of Meteorology (2022) State of the Climate 2022. CSIRO and Bureau of Meteorology, Australia.
Cwlth TSSC (Commonwealth Threatened Species Scientific Committee) (2018) Conservation Advice Wollemia nobilis Wollemi Pine. Department of the Environment and Energy. Accessed 19 May 2023.
Davison EM (2011) How do Phytophthora spp. de Bary kill trees?. New Zealand Journal of Forestry Science 41 (Suppl.), S25–S37.
Dettmann ME & Jarzen DM (2000) Pollen of extant Wollemia (Wollemi Pine) and comparisons with pollen of other extant and fossil Araucariaceae, In: Harly MM, Morton CM & Blackmore (eds) Pollen and Spores: Morphology and Biology. Royal Botanic Gardens, Kew. 197–203.
DCCEEW (Department of Climate Change, Energy, the Environment and Water) (2022) Threatened Species Strategy Action Plan 2022–2032. Department of Climate Change, Energy, the Environment and Water, Canberra.
DoEE (Department of the Environment and Energy) (2018) Threat abatement plan for disease in natural ecosystems caused by Phytophthora cinnamomi. Australian Government, Canberra.
Dowdy A et al. (2015) East Coast Cluster Report, Climate Change in Australia Projections for Australia’s Natural Resource Management Regions: Cluster Reports, eds. Ekström M et al. CSIRO and Bureau of Meteorology, Australia.
Escapa IH & Catalano SA (2013) Phylogenetic analysis of Araucariaceae: integrating molecules, morphology, and fossils. International Journal of Plant Sciences 174 (8), 1153–1170.
Everham EM & Brokaw NV (1996) Forest damage and recovery from catastrophic wind. The Botanical Review 62, 113–185.
Fensom G & C Offord (1998) Propagation of Wollemi Pine (Wollemia nobilis). Combined Proceedings International Plant Propagators’ Society 47, 66–67.
Gilmore S & KD Hill (1997) Relationships of the Wollemi Pine (Wollemia nobilis) and a molecular phylogeny of the Araucariaceae. Telopea 7 (3), 275–291.
Griffith MP, Beckman E, Calicrate T, Clark JR, Clase T, Deans S, Dosmann M, Fant J, Gratacos X, Havens K, Hoban S, Lobdell M, Jimenez-Rodriguez F, Kramer A, Lacy R, Magellan T, Maschinski Joyce, Meerow AW, Meyer A, Sanchez V, Spence E, Toribio P, Walsh S, Westwood M & Wood J (2019) Toward the metacollection: Safeguarding plant diversity and coordinating conservation collections. Botanic Gardens Conservation International-US.
Griffith MP, Clase T, Toribio P, Piñeyro YE, Jimenez F, Gratacos X, Sanchez V, Meerow A, Meyer A, Kramer A & Fant J (2020) Can a botanic garden metacollection better conserve wild plant diversity? A case study comparing pooled collections with an ideal sampling model. International Journal of Plant Sciences 181 (5), 485–496.
Greenfield A, McPherson H, Auld T, Delaney S, Offord CA, Van Der Merwe M, Yap JYS & Rossetto M (2016) Whole-chloroplast analysis as an approach for fine-tuning the preservation of a highly charismatic but critically endangered species, Wollemia nobilis (Araucariaceae). Australian Journal of Botany 64 (8), 654–658.
Harden GJ (1990) Araucariaceae, In: Harden GJ (ed) Flora of New South Wales, Vol. 1. University of New South Wales Press, Kensington, Sydney.
Haworth M, Elliott-Kingston C & McElwain JC (2011) The stomatal CO 2 proxy does not saturate at high atmospheric CO 2 concentrations: evidence from stomatal index responses of Araucariaceae conifers. Oecologia, 167 (1), 11–19.
Heady R (2002) Micro-structure of Araucariaceae, In: Dargavel J, Gaughwin D & Libbis B (eds) Australia’s Ever-changing Forests V, Proceedings of the Fifth National Conference on Australian Forest History. Centre for Resource and Environmental Studies, ANU, Canberra. Pp. 90–101.
Heady RD, Banks JG & Evans PD (2002) Wood anatomy of Wollemi pine (Wollemia nobilis, Araucariaceae). IAWA Journal 23 (4), 339–357.
Heady RD & Burrows GE (2008) Features of the secondary xylem that facilitate branch abscission in juvenile Wollemia nobilis. IAWA Journal 29 (3), 225–236.
Hill K (1995) The Wollemi Pine: watch out, look around you. The Gardens 27, 8–9.
Hill K (1996) The Wollemi Pine: discovering a living fossil. Nature and Resources 32 (1), 20–25.
Hill KD (1997) Architecture of the Wollemi pine (Wollemia nobilis, Araucariaceae), a unique combination of model and reiteration. Australian Journal of Botany 45(5), 817–826.
IUCN Standards and Petitions Committee (2024) Guidelines for Using the IUCN Red List Categories and Criteria. Version 16.
Jones WG, Hill KD & Allen JM (1995) Wollemia nobilis, a new living Australian genus and species in the Araucariaceae. Telopea 6, 173–176.
Kershaw P & Wagstaff B (2001) The southern conifer family Araucariaceae: history, status, and value for paleoenvironmental reconstruction. Annual Review of Ecology and Systematics 32, 397–414.
Lange RT (1982) Australian tertiary vegetation, In: Smith JMB (ed) A History of Australian Vegetation. McGraw Hill Book Co., Australia.
Lewis JD, Phillips NG, Logan BA, Smith RA, Aranjuelo I, Clarke S, Offord CA, Frith A, Barbour M, Huxman T & Tissue DT (2015) Rising temperature may negate the stimulatory effect of rising CO2 on growth and physiology of Wollemi pine (Wollemia nobilis). Functional Plant Biology 42 (9), 836–850.
Liu N, Zhu Y, Wei Z, Chen J, Wang Q, Jian S, Zhou D, Shi J, Yang Y & Zhong Y (2009) Phylogenetic relationships and divergence times of the family Araucariaceae based on the DNA sequences of eight genes. Chinese Science Bulletin 54 (15), 2648–2655.
Mackenzie BDE (2021) The intergenerational effort to secure a future for the iconic Wollemi Pine. National Parks Association of NSW. Accessed 7 December 2023.
Mackenzie BDE, Clarke SW, Zimmer HC, Liew ECY, Phelan MT, Offord CA, Menke LK, Crust DW, Bragg J, McPherson H, Rossetto M, Coote DM, Yap J-YS & Auld TD (2021) Ecology and conservation of a living fossil: Australia’s Wollemi Pine (Wollemia nobilis), In: DellaSala D & Goldstein M (eds) Imperiled: The Encyclopedia of Conservation.
Mackenzie BDE & Auld TD (in press) Wollemia nobilis. The IUCN Red List of Threatened Species 2024.
Macphail M, Hill K, Partridge A, Truswell E & Foster C (1995) Wollemi Pine - old pollen records for a newly discovered genus of gymnosperm. Geology Today 11 (2), 48–50.
Macphail M, Carpenter RJ, Iglesias A & Wilf P (2013) First evidence for Wollemi pine-type pollen (Dilwynites: Araucariaceae) in South America. PLoS One 8 (7), e69281.
Macphail M & Carpenter RJ (2014) New potential nearest living relatives for Araucariaceae producing fossil Wollemi Pine-type pollen (Dilwynites granulatus WK Harris, 1965). Alcheringa: An Australasian Journal of Palaeontology 38 (1), 135–139.
Martyn Yenson AJ , Offord CA, Meagher PM, Auld T, Bush D, Coates DJ, Commander LE, Guja LK, Norton SL, Makinson RO, Stanley R, Walsh N, Wrigley D, Broadhurst L (2021) Plant Germplasm Conservation in Australia: strategies and guidelines for developing, managing and utilising ex situ collections. Third edition. Australian Network for Plant Conservation, Canberra.
McGee PA, Bullock S & Summerell BA (1999) Structure of mycorrhizae of the Wollemi pine (Wollemia noblis) and related Araucariaceae. Australian Journal of Botany, 47 (1), 85–95.
McLoughlin S & Vajda V (2006) The Wollemi Pine and the fossil record. Australian Age of Dinosaurs 4, 54–55.
Nash S & Ravallion J (1998) Wollemi Pine (Wollemia nobilis) Recovery Plan - 1997-2002. NSW National Parks and Wildlife Service, Hurstville.
Ng MCH, Tran VH, Duke RK, Offord CA, Meagher PF, Cui PH, Duke CC (2024) Lipid Profile of Fresh and Aged Wollemia nobilis Seeds: Omega-3 Epoxylipid in Older Stored Seeds. Lipidology, 1 (2), 92–104. https://doi.org/10.3390/lipidology1020007
NSW DCCEEW (Department of Climate Change, Energy, the Environment and Water) (2024) Aboriginal joint management model consultation. Accessed 30 May 2024.
NSW DEC (Department of Environment and Conservation) (2006a) Wollemia nobilis (Wollemi Pine) Recovery Plan. Accessed 19 May 2023.
NSW DEC (Department of Environment and Conservation) (2006b). Critical Habitat determination for the Wollemi Pine (Wollemia nobilis) Araucariaceae - A determination under Part 3 of the Threatened Species Conservation Act 1995. NSW Department of Environment and Conservation, Hurstville NSW.
NSW DPE (Department of Planning and Environment) (2022) Wollemi Pine Conservation Action Plan. Department of Planning and Environment. Accessed 10 October 2022.
NSW NPWS (NSW National Parks and Wildlife Service) (2000) The Greater Blue Mountains Area World Heritage Nomination. NSW National Parks and Wildlife Service.
NSW NPWS (NSW National Parks and Wildlife Service) (2009) Greater Blue Mountains World Heritage Area Strategic Plan. NSW Department of Environment and Climate Change.
NSW NPWS (NSW National Parks and Wildlife Service) (2022) Conservation action plan: Wollemi Pine (Wollemia nobilis). NSW National Parks and Wildlife Service. Accessed 19 May 2023.
NSW NPWS (NSW National Parks and Wildlife Service) (2023) Assets of Intergenerational Significance. NSW National Parks and Wildlife Service. Accessed 19 May 2023.
NSW NPWS (NSW National Parks and Wildlife Service (2024) Aboriginal engagement to guide strategic management of the Greater Blue Mountains Area World Heritage property. NSW National Parks and Wildlife Service. Accessed 30 May 2024.
NSW TSSC (NSW Threatened Species Scientific Committee) (2015) Final determination: Wollemi Pine (Wollemia nobilis). Accessed 18 May 2023.
Offord CA (2011) Pushed to the limit: consequences of climate change for the Araucariaceae: a relictual rain forest family. Annals of Botany 108, 347–357.
Offord CA, Errington G & Cuneo P (1996) Report to the management committee work at Mount Annan Botanic Garden. Unpublished report, July 1996.
Offord CA & Meagher PF (2001) Effects of temperature, light and stratification on seed germination of Wollemi Pine (Wollemia nobilis Araucariaceae). Annals of Botany 49 (6), 699–704.
Offord CA, Meagher PF, & Zimmer HC (2014) Growing up or growing out? How soil pH and light affect seedling growth of a relictual rainforest tree. AoB Plants 6, plu011.
Offord CA, Porter CL, Meagher PF & Errington G (1999) Sexual Reproduction and early plant growth of the Wollemi Pine (Wollemia nobilis), a rare and threatened Australian conifer. Annals of Botany 84, 1–9.
Offord CA & Zimmer HC (2023) Home gardens contribute to conservation of the critically endangered Wollemi Pine: Evaluation of a botanic garden‐led horticultural release programme. Plants, people, planet 6 (1), 116–127.
Owens JN, Catalano GL & Aitken-Christie J (1997) The reproductive biology of Kauri (Agathis Australia). IV. Late embryogeny, histochemistry, cone and seed morphology. International Journal of Plant Sciences 158 (4), 395–407
Peakall R, Ebert D, Scott LJ, Meagher PF & Offord CA (2003) Comparative genetic study confirms exceptionally low genetic variation in the ancient and endangered relictual conifer, Wollemia nobilis (Araucariaceae). Molecular Ecology 12 (9), 2331–2343.
Pole M (2008) The record of Araucariaceae macrofossils in New Zealand. Alcheringa 32 (4), 405–426.
Puno VI, Laurence MH, Guest DI & Liew ECY (2015) Detection of Phytophthora multivora in the Wollemi Pine site and pathogenicity to Wollemia nobilis. Australasian Plant Pathology 44 (2), 205–215.
Ravallion J (2000) New Wollemi Pine site found. Danthonia 9 (3), 5.
Rigg JL, Offord CA, Singh BK, Anderson I, Clarke S & Powell JR (2016a) Soil microbial communities influence seedling growth of a rare conifer independent of plant–soil feedback. Ecology 97 (12), 3346–3358.
Rigg JL, Offord CA, Singh BK, Anderson IC, Clarke S & Powell JR (2016b) Variation in soil microbial communities associated with critically endangered Wollemi Pine affects fungal, but not bacterial, assembly within seedling roots. Pedobiologia, 59 (1-2), 61–71.
Rigg JL, Offord CA, Zimmer H, Anderson IC, Singh BK & Powell JR (2017) Conservation by translocation: establishment of Wollemi Pine and associated microbial communities in novel environments. Plant and Soil, 411(1-2), 209–225.
Salleh A (2005) News in science: Wollemi Pine infected by fungus. ABC Science. Accessed 19 May 2023.
Setoguchi H, Asakawa Osawa T, Pintaud JC, Jaffré T & Veillon JM (1998) Phylogenetic relationships within Araucariaceae based on rbcL gene sequences. American Journal of Botany 85 (11), 1507–1516.
Seneviratne S, Nicholls N, Easterling D, Goodess CM, Kanae S, Kossin J, Luo Y, Marengo J, McInnes K, Rahimi M, Reichstein M, A. Sorteberg, C. Vera & X. Zhang (2012) Changes in climate extremes and their impacts on the natural physical environment, In: Field CB, Barros V, Stocker TF, Qin D, Dokken DJ, Ebi KL, Mastrandrea MD, Mach KJ, Plattner G-K, Allen SK, Tignor M, & Midgley PM (eds) A Special Report of Working Groups I and II of the Intergovernmental Panel on Climate Change (IPCC). Cambridge University Press, Cambridge, UK, and New York, NY, USA, pp. 109–230.
Seyfullah LJ, Coiro M & Hofmann CC (2023) In situ pollen diversity in the relict conifer Wollemia nobilis. Review of Palaeobotany and Palynology 309, 104816.
Stefanović S, Jager M, Deutsch J, Broutin J & Masselot M (1998) Phylogenic relationships of conifers inferred from partial 28S rRNA gene sequences. American Journal of Botany 85, 688–697.
Stevenson DW, Ramakrishnan S, de Santis Alves C, Coelho LA, Kramer M, Goodwin S, Ramos OM, Eshel G, Sondervan VM, Frangos S, Zumajo-Cardona C, Jenike K, Ou S, Wang X, Lee YP, Loke S, Rossetto M, McPherson H, Nigris S, Moschin S, Little DP, Katari MS, Varala K, Kolokotronis S-O, Ambrose B, Croft LJ, Coruzzi GM, Schatz M, McCombie WR & Martienssen RA (2023 preprint) The genome of the Wollemi pine, a critically endangered “living fossil” unchanged since the Cretaceous, reveals extensive ancient transposon activity. bioRxiv.
Strobel GA, Hess WM, Li JY, Ford E, Sears J, Sidhu RS & Summerell B (1997) Pestalotiopsis guepinii, a Taxol-producing Endophyte of the Wollemi Pine, Wollemia nobilis. Australian Journal of Botany 45, 1073–1082.
Thomas P (2011) Wollemia nobilis. The IUCN Red List of Threatened Species 2011: e.T34926A9898196. Accessed on 18 May 2023.
Tomlinson PB & Huggett BA (2011) Partial shoot reiteration in Wollemia nobilis (Araucariaceae) does not arise from ‘axillary meristems’. Annals of Botany 107 (6), 909–916.
Tomlinson PB & Huggett BA (2011) Axillary meristems in the Wollemi Pine: a response to Geoffrey E. Burrows. Annals of Botany 109 (1), 3–4.
Tomlinson PB & Murch SJ (2009) Wollemia nobilis (Araucariaceae): branching, vasculature and histology in juvenile stages. American Journal of Botany 96 (10), 1787–1797.
Trueman S J & Peters RF (2006) Propagation of Wollemi pine from tip cuttings and lower segment cuttings does not require rooting hormones. Scientia Horticulturae 109 (4), 394–397
Trueman SJ, Pegg G & King J (2007) Domestication for conservation of an endangered species: the case of the Wollemi Pine. Tree Forestry Science and Biotechnology 1, 1–10
Ukkola AM, De Kauwe MG, Roderick ML, Abramowitz G & Pitman AJ (2020) Robust future changes in meteorological drought in CMIP6 projections despite uncertainty in precipitation. Geophysical Research Letters, 47(11), e2020GL087820.
van Oldenborgh GJ, Krikken F, Lewis S, Leach NJ, Lehner F, Saunders KR, van Weele M, Haustein K, Li S, Wallom D, Sparrow S, Arrighi J, Singh RK, van Aalst MK, Philip SY, Vautard R, and Otto FEL (2021) Attribution of the Australian bushfire risk to anthropogenic climate change. Natural Hazards and Earth System Sciences Discussions 21, 941–960.
Whitmore T (1989) Canopy gaps and the two major groups of forest trees. Ecology 70 (3), 536–538.
Yap JYS, Rohner T, Greenfield A, Van Der Merwe M, McPherson H, Glenn W, Kornfeld G, Marendy E, Pan AY, Wilton A, Wilkins MR, Rossetto M & Delaney SK (2015) Complete chloroplast genome of the Wollemi Pine (Wollemia nobilis): structure and evolution. PloS one 10 (6), e0128126.
Zimmer HC, Auld TD, Benson J & Baker PJ (2014) Recruitment bottlenecks in the rare Australian conifer Wollemia nobilis. Biodiversity and Conservation 23, 203–215.
Zimmer HC, Meagher PF, Auld TD, Plaza J & Offord CA (2015a) Year-to-year variation in cone production in Wollemia nobilis (Wollemi Pine). Cunninghamia 15, 79–85.
Zimmer HC, Brodribb TJ, Delzon S & Baker PJ (2015b) Drought avoidance and vulnerability in the Australian Araucariaceae. Tree Physiology 36 (2), 218–228.
Zimmer HC, Auld TD, Hughes L, Offord CA & Baker PJ (2015c) Fuel flammability and fire responses of juvenile canopy species in a temperate rainforest ecosystem. International Journal of Wildland Fire 24 (3), 349–360.
Zimmer HC, Offord CA, Auld TD & Baker PJ (2016) Establishing a wild, ex situ population of a critically endangered shade-tolerant rainforest conifer: a translocation experiment. PloS one 11 (7), e0157559.
Research into the Wollemi Pine has been ongoing since its discovery in 1994. Research has been conducted both in the wild and using ex situ material. Major themes of research and key publications, in approximate order of occurrence, are described below. Publications particularly relevant to the conservation management of the Wollemi Pine are highlighted in bold font.
Wollemi pine anatomy and morphology:
i. Formal species circumscription (Jones et al. 1995).
ii. Branching and coppicing habit (Hill 1997).
iii. Leaf and branching anatomy:
Leaf anatomy (Burrows and Bullock 1999), leaf axil anatomy (Burrows 1999).
Branch abscission. The Wollemi Pine has a very unusual branch connection which is associated with branch abscission (Burrows et al. 2007; Heady et al. 2008).
Branching and vasculature of the Wollemi Pine (Tomlinson & Murch 2009).
Debate on axillary meristems (Burrows et al. 2003, Tomlinson and Huggett 2011, 2012).
iv. Wood anatomy (Heady et al. 2000)
Habitat:
i. Vegetation associated with the Wollemi Pine (Benson and Allen 2007).
Phylogeny:
i. Context within Araucariaceae (Gilmore and Hill 1997).
ii. Revised by Stefanovic et al. (1998).
iii. Revised by Setoguchi et al. (1998).
iv. Revised by Liu et al. (2009).
v. Revised by Escapa and Catalano (2013)
Fossil evidence linked to the Wollemi Pine:
i. Pollen and palynology (Macphail et al. 1995; Hill 1996, Dettmann & Jarzen 2000), and links between Dilwynites fossil pollen and Araucariaceae other than Wollemia (Macphail et al. 2013; Macphail & Carpenter 2014; Seyfullah et al. 2023).
ii. Comparison to Creteacous plant fossils (Chambers et al. 1998).
iii. Macrofossils (Pole 2008).
Recruitment, reproduction and seed ecology:
i. Reproductive biology and ecology and early plant growth (Offord et al. 1999).
ii. Seed ecology and germination requirements (Offord & Meagher 2001).
iii. Inter-annual variation in cone production (Zimmer et al. 2015a).
iv. Population structure and recruitment limitation (Zimmer et al. 2014).
Tree rings/dendrochronology:
i. Pilot study on five trees by Banks (2002).
ii. Follow-up study by Baker and Simkin (2010) who highlight the potential for chronologies of <300 years, and the challenges associated with missing rings and ring wedging.
Mycorrhizae, soil microbiota, endophytes and soil pathogens:
i. Pestalotiopsis guepinii – a taxol producing endophyte of the Wollemi Pine (Strobel et al. 1997).
ii. Mycorrhizae of the Wollemi Pine (McGee et al 1999).
iii. Soil microbiota associated with the Wollemi Pine (Rigg et al. 2016a; Rigg et al. 2016b).
iv. The importance of Phytophthora cinnamomi and Botryosphaeria as pathogens of the Wollemi Pine (Bullock et al. 2000).
v. Phytophthora multivora as a pathogen of the Wollemi Pine (Puno et al. 2015).
Genetic variation in Wollemi Pine:
i. Extremely low genetic variation of the Wollemi Pine (Peakall et al. 2003).
ii. Chloroplast genome of the Wollemi Pine (Yap et al. 2015).
iii. Chloroplast DNA confirms low genetic variation (Greenfield et al. 2016).
iv. The genome of the Wollemi Pine (Stevenson et al. 2023 preprint).
Physiological tolerances of the Wollemi Pine, and predictions for response to changing climate:
i. Response to high temperatures (Offord 2011).
ii. Response to CO2 (Haworth et al. 2011).
iii. Response to temperature and CO2 (Lewis et al. 2015).
iv. Drought intolerance (Zimmer et al. 2015b).
v. Resprouting response to fire and litter flammability (Zimmer et al. 2015c).
Propagation and cultivation of the Wollemi Pine:
i. Propagation of juvenile orthotropic shoots (Fensom and Offord 1998).
ii. Wollemi Pine growth responses to soil pH and light (Offord et al. 2014).
iii. Factors affecting establishment and growth in cultivation around the globe (Offord & Zimmer 2023).
Translocation:
i. Establishment of a pilot translocation site (Zimmer et al. 2016).
ii. Establishment of Wollemi Pine microbial communities in a translocated stand (Rigg et al. 2017).
Other:
i. Essential oil of Wollemi Pine (Brophy et al. 2000).
Performance criterion 10.1.1: The known stands of the Wollemi Pine are protected from damage by visitation to the stands. Enforcement of area closures, under the NPW Act Land Management Regulations and regulations in accordance with critical habitat occur.
Access to the wild Wollemi Pine stands continues to be highly restricted for both NSW DCCEEW staff and the public to minimise the risk to the remaining plants and their habitat. It is illegal to visit the Wollemi Pine wild population without authorisation from NPWS. The Wollemi Pine Access Strategy outlines the protocols for site access and restricts access only to essential personnel authorised by NSW DCCEEW. These measures have been further codified in the Wollemi National Park Plan of Management (2001), the Wollemi National Park Fire Management Plan (2006) and the Wollemi Pine Conservation Action Plan (2021).
Authorised visits to the wild stands for research purposes have also been monitored and approved by the Wollemi Pine Recovery Team. Only essential on-site work has been authorised. Wherever possible, use of plant material from the off-site populations at the ABGMA and the BMBGMT is prioritised over visits to the wild population. A registry of all approved site visits is maintained by NPWS. Illegal visitation has been minimal and on-ground site surveillance is continuing.
The declaration of Critical Habitat for the Wollemi Pine was made in 2007 under the NSW Threatened Species Conservation Act 1995 (TSC Act) (now repealed). The identification report for the declaration of Critical Habitat for the Wollemi Pine also included regulations in accordance with Section 51 of the TSC Act to prohibit access to the critical habitat area and penalties for failure to comply with a direction given by a designated officer to leave the critical habitat area. Upon the repeal of the TSC Act, the Critical Habitat for the Wollemi Pine became an AOBV under the BC Act. The regulations to prohibit access to the declared area were substantially replicated in the Biodiversity Conservation Regulation 2017.
The Wollemi Pine was declared an AIS under the NPW Act in 2021 and a CAP was developed. The activities described in the Wollemi Pine CAP include “Implement law enforcement and compliance action to prevent unauthorised access, to the extent practicable” and “Ensure all authorised access occurs in accordance with risk mitigation protocols which avoid significant impacts arising from trampling of plants, compaction and erosion of soils and the introduction and spread of pathogens.”
Performance criterion 10.1.2 The known stands of the Wollemi Pine are protected from the detrimental impacts of pathogens or other threats to plant health.
Phytophthora cinnamomi and P. multivora have been detected in Stand 1. The sources of introduction are unknown but are suspected to be the result of unauthorised visitation. Strict quarantine measures (including the Site Hygiene Protocol) have been, and will continue to be, enforced during all authorised site visits. The Site Hygiene Protocol has been updated and expanded since the previous recovery plan.
Performance criterion 10.1.3 A monitoring program is designed and implemented, and treatments are investigated and used, leading to the extent of P. cinnamomi in the site being controlled, further infections being halted and infected trees being effectively treated.
Monitoring of the Phytophthora spp. infection in Stand 1 is ongoing. Repeated soil testing has revealed the detectability of the pathogens along fixed transects varies over time. It is noted that absences may sometimes be detection failure, rather than true absences. Ridomil fungicide treatments have been discontinued following review of the wider environmental impacts associated with the application of the product and limited suppression success.
Wollemi Pine health monitoring is also ongoing, particularly around trees which have shown symptoms of Phytophthora infection. A non-destructive method of using phosphonate to treat Phytophthora infection has been developed based on glasshouse trials but has yet to be applied and tested on wild trees. The method involves painting phosphonate solution in conjunction with a penetrant onto the bark tissue, which allows chemical uptake by the tree. This reduces disease severity in infected trees and protects healthy trees from infection. Because the introduced chemical triggers energy-demanding defence mechanisms within treated trees, the treatment is only to be applied on trees that are free of, or have recovered from, severe stress ,such as extreme environmental conditions or fire damage.
Performance criterion 10.1.4 The stands of the Wollemi Pines are protected from the detrimental impacts of pollution, flooding, weed infestation, sedimentation and other adverse changes to the hydrology of the catchment wild population.
The wild Wollemi Pine population has been protected from pollution and weed infestation. Several flood events have occurred since the last recovery plan with no major impacts on the population or its habitat. Control of invasive weeds within the catchment is undertaken on a routine basis.
Performance criterion 10.1.5 The risk of damage to Wollemi Pine stands from wildfire and bushfire management activities will be minimised.
A Wollemi Pine Fire Management Strategy (FMS) was prepared in 2022 as a requirement of the CAP. Proposed actions under the FMS include continuation of the Rapid Aerial Response Team program for rapid suppression of fires within the Wollemi Pine catchment, in-grove protections in response to anticipated catastrophic fire conditions, hazard reduction burning at a landscape scale to limit bushfire spread and intensity, and inclusion of species experts in fire incident management teams.
Performance criterion 10.1.6 Guidelines for translocation of Wollemi Pine are prepared.
Guidelines for translocation were prepared as part of the 2019 translocations in Wollemi National Park. These will be updated as part of a review of the translocation program.
Performance criterion 10.1.7 Critical Habitat and associated regulations are declared and enforced as required.
A 5,000 hectare area of Wollemi National Park was declared as an AIS in 2021, supporting the declaration of the habitat of the Wollemi Pine as Critical Habitat in December 2007 under the TSC Act. Although the TSC Act has been repealed, the Critical Habitat for the Wollemi Pine became an AOBV upon enactment of the BC Act. The CAP describes the conservation activities required to control, abate or mitigate key risks to the environmental values of the AIS, and maintain, restore and remediate the environmental values of the AIS.
Performance criterion 10.2.1 Aspects of the stand dynamics of the Wollemi Pine are documented and the age structures of the standing trunks in known stands are estimated. Ecological data gained is used to modify the management program outlined in Section 10.1 and assists in the tasks outlined in Section 10.3 and 10.4.
The stand dynamics of the Wollemi Pine were documented in a peer reviewed scientific paper, which described evidence for the Wollemi Pine having a recruitment strategy of maintaining a juvenile bank (Zimmer et al. 2014). This study highlighted the rarity of recruitment events, and hence the importance of protecting all seedlings and juveniles. Size structures of the standing trunks (diameters at breast height) were documented in 2003 and 2013. Growth rates have not yet been reliably estimated; hence, ages have not been estimated.
Monitoring has been ongoing at the wild stands since 1995. At Stand 1, each individual seedling, juvenile and mature tree stem greater than or equal to 2 cm DBH was tagged. Results of pre-fire monitoring of seedling and juvenile growth and survival at Stand 1 are presented in Zimmer et al. (2014). The majority of seedlings were killed in the 2019–20 fire and tags on individual coppice stems at Site 1 were lost. However, since 2020, fire-resistant tags have been applied to all stems greater than or equal to 2 m tall on all accessible juvenile and mature trees across all four wild sites, the relative positions of stems on individual trees mapped, and stem height, DBH and reproductive status recorded (Mackenzie, Clarke and Auld, unpubl. data). Canopy photography is also utilised to monitor tree health and major changes in canopy cover and structure.
Performance criterion 10.2.2 The fire history of the sites and impact of fire, rock-fall and wind shake on Wollemi Pines is examined. Key gap forming processes are identified. This information is used to modify existing management practices outlined under Section 10.1.
Field surveys of the wild Wollemi Pine population after the 2019–20 bushfires revealed that most adult trees survived the fires with their upper canopies intact (Mackenzie et al. 2021). Of the fire-affected trees greater than or equal to 2 m tall, two-thirds suffered partial canopy loss and one-third (predominantly juvenile trees) were completely top-killed (Mackenzie et al. unpubl. data). Of these top-killed individuals, approximately one-third were killed outright with the remainder (c. 20% of the post-fire population) reduced to vulnerable basal resprouts (Mackenzie et al. unpubl. data). The pre-fire bank of seedlings and smaller juveniles less than 2m tall has been 92–95% eliminated with only 4–7% of pre-fire individuals successfully resprouting (Mackenzie et al. unpubl. data). However, the juvenile bank is expected to re-establish from seed over the next 20-30 years (Mackenzie et al. 2021).
Earlier experimental studies of the impact of fire (under nursery conditions) on Wollemi Pines and three co-dominant rainforest angiosperms found that that cultivated cuttings of all four study species had the capacity to resprout after a single, low intensity fire.
There have been no further studies into rock fall or windshake.
Field observations suggest fire, rock fall and wind are likely to be the main canopy gap forming processes, along with natural senescence of dominant trees.
Performance criterion 10.2.3 Qualified individual/s are found to initiate a study/studies to increase understanding of evolutionary history of Wollemi Pine.
The evolutionary history of the Wollemi Pine has been well studied, including by Gilmore and Hill (1997), Stefanovic et al. (1998), Setoguchi et al. (1998), Liu et al. (2009) and Escapa & Catalano (2013).
Performance criterion 10.2.4 New techniques for the analysis of population genetics are investigated and, where appropriate, applied.
The chloroplast genome of the Wollemi Pine has been sequenced (Yap et al. 2015). Whole-chloroplast analysis has revealed low but detectable genetic diversity (Greenfield et al. 2016). More refined population genetic studies, based on reduced representation genome sequencing approaches are currently in progress, but preliminary analyses have detected low-level variation within and between subpopulations (Bragg et al. unpubl., Stevenson et al. 2023 preprint). These data are being used to guide the development of ex situ collections and translocated populations to maximize their genetic diversity (Mackenzie et al. 2021).
Performance criterion 10.2.5 Embryology and cone production cycles are better understood providing information for establishing evolutionary relationships within Araucariaceae and enabling estimation of seed production in different years. Knowledge gained is used to modify the management program outlines in Section 10.1.
Inter-annual variation in cone production was described by Zimmer et al. (2015a). Evolutionary relationships within the Araucariaceae have been explored, mainly using genetic analysis (see Performance criterion 2.3). Further research into the embryology of the Wollemi Pine is required.
Performance criterion 10.2.6 Knowledge of species associated with the Wollemi Pine and its habitat is gained and this information is used in the management of the wild population (Action10.1).
The rainforest community of the Wollemi Pine was described in detail by Benson and Allen (2007).
The soil microbiota associated with the Wollemi Pine was described by Rigg et al. (2016a).
Performance criterion 10.2.7 Potential habitat is identified and field searched.
Potential habitat for Wollemi Pine has been modelled, based on environmental characteristics such as geology, climate, vegetation type and fire history. However, the highly remote and dissected nature of the Wollemi National Park landscape meant that there were limited on-ground data to inform these models, leading to uncertainty in the reliability of predicted habitat.
Extensive helicopter and ground surveys have been undertaken within Wollemi National Park, and no additional stands have been found.
The living collection at ABGMA is a key component of the Wollemi Pine conservation program, providing important insurance against loss of genetic diversity in the wild population, and enabling a wide variety of ex situ research. However, like the wild population, a single, concentrated collection is highly vulnerable to stochastic events. Hence, propagation is ongoing to support the maintenance of a comprehensive backup in-ground collection at BMBGMT, and global distribution of a genetically diverse, dispersed living collection under the Botanic Gardens Conservation International (BGCI) metacollections program (Griffith et al., 2019) to further back-up and protect this vital resource.
Performance criterion 10.3.1 Material is available to allow: commercial propagation; dispersal to other conservation agencies, principally botanic gardens; establishment of a representative off-site collections at BGT; any potential future translocation; and scientific research.
A potted ex situ living collection has been established and maintained at ABGMA. Work is underway to create a comprehensive in-ground backup of this collection at BMBGMT, and a smaller collection is also maintained at the Botanic Gardens of Sydney BMBGMT. Material has been made available to support commercial propagation and distribution; dispersal to other conservation agencies (principally botanic gardens); three recent (2019, 2021 and 2024) translocations into Wollemi National Park (Mackenzie et al. 2021; Mackenzie et al. unpubl. data); and ongoing scientific research.
Performance criterion 10.3.2 Determination of the optimum conditions for seed germination and storage is achieved, providing a means of low-cost off-site conservation. This knowledge is used to inform management of the wild population.
Research has been undertaken into the optimum conditions for seed germination (Offord and Meagher 2001). Wollemi Pine seeds have been banked at the ABGMA PlantBank and research into optimum conditions for storage is ongoing. As more Wollemi Pine individuals in the BMBGMT in-ground collection reach maturity, they will provide the most genetically diverse source of seed available (given collocation of trees from all four wild stands), reducing the need to collect seed from the wild population.
Performance criterion 10.3.3 The optimum conditions for the cultivation of the Wollemi Pine are determined and knowledge gained is used to inform management of the wild population.
An extensive program of research into the propagation and horticulture of the Wollemi Pine has, in large part, been led by the RBGDT. The Wollemi Pine has been successfully germinated from seed (Offord & Meagher 2001), propagated from cuttings (Fensom & Offord 1998, Trueman & Peters 2006) and grown in cultivation (Offord et al. 1999; Offord 2011; Offord et al. 2014). Multiple studies have defined optimal conditions for Wollemi Pine growth in cultivation including:
Reproductive biology and ecology and early plant growth (Offord et al. 1999).
Temperature tolerances (Offord 2011).
Response to CO2 (Haworth et al. 2011).
Response to temperature and CO2 (Lewis et al. 2015).
Intolerance of drought (Zimmer et al. 2015b).
Wollemi Pine growth responses to soil pH and light (Offord et al. 2014).
Performance criterion 10.4.1 Community appreciation of and support for the conservation and protection of the Wollemi Pine is enhanced. This has been measured by surveys of audiences of Wollemi Pine presentations / lessons conducted by RBGDT Community Education, off site collections are planted and interpreted, Wollemi Pine website is maintained, exhibitions at field-days around Wollemi National Park are attended and educational information for general release is produced.
While there has been no formal survey of audiences at Wollemi Pine presentations, observations of researchers and Recovery Team members suggest that interest and support for the Wollemi Pine remains strong. During the 2019–20 bushfire season, the interest in the Wollemi Pines and potential impacts of the fires was intense, and this interest continued in the period after the fires. Citizen Science projects including ‘I Spy a Wollemi’ raised community awareness and support for the species both nationally and internationally
Ex situ collections have been planted at ABGMA and BMBGMT with associated interpretative material. The RBGDT Wollemi Pine webpages were updated in 2024 and remain important sources of information on the Wollemi Pine. NPWS also maintain several webpages dedicated to the Wollemi Pine conservation project, including a series of educational videos. Since the last recovery plan, there have been myriad TV news segments, newspaper and magazine articles, documentaries, radio interviews and podcasts on the Wollemi Pine.
Performance criterion 10.5.1 The known stands of Wollemi Pine are protected from damage caused by illegal visitation. Revenue from the commercialisation program is returned to the conservation of the Wollemi Pine.
A commercialisation strategy was developed to manage the release of the plants into cultivation. This strategy was launched in October 2005 at an international auction conducted by Sotherby’s at the BGOS. The strategy involved the development of a commercial release scheme that is sensitive to the conservation concerns of this and other Australian species, ensuring minimal material is collected from the wild, discouraging illegal collection, and communicating the importance of conservation. Upon commercial release of Wollemi Pine, messages regarding plant conservation were provided with packaging, printed, and web-based documentation, and other publicity efforts, in concert with the community relations strategy. Wollemi Pines continue to be sold commercially, although the Wollemi Pine Recovery Team and RBGDT have little control over the market because of the ability for suppliers and individuals to propagate their own Wollemi Pines.
Communication around the Wollemi Pine maintains robust messaging against illegal visitation. A proportion of the initial revenue from the commercialisation program was returned to the conservation of the Wollemi Pine. However, relationships with the commercial sector ceased in 2010 and royalties no longer contribute to conservation activities (B. Summerell, pers. comm.).














