
Environment Protection and Biodiversity Conservation (National Recovery Plan for the Water Mouse – Xeromys myoides) Instrument 2023
We jointly make this recovery plan under subsection 269A(3) of the Environment Protection and Biodiversity Conservation Act 1999.
Dated 16.11.2023
Tanya Plibersek
Tanya Plibersek
Minister for the Environment and Water (Commonwealth)
Dated 4.09.2023
Leanne Linard
Leanne Linard
Minister for the Environment and the Great Barrier Reef (Queensland)
Dated 11.09.2023
Lauren Moss
Lauren Moss
Minister for Environment, Climate Change and Water Security (Northern Territory)
National Recovery Plan for the Water Mouse
(Xeromys myoides)

1 December 2022
The Species Profile and Threats Database page linked to this recovery plan is obtainable from: http://www.environment.gov.au/cgi-bin/sprat/public/publicspecies.pl?taxon_id=66
© Commonwealth of Australia 2023
Ownership of intellectual property rights
Unless otherwise noted, copyright (and any other intellectual property rights) in this publication is owned by the Commonwealth of Australia (referred to as the Commonwealth).
Creative Commons licence
All material in this publication is licensed under a Creative Commons Attribution 4.0 International Licence except content supplied by third parties, logos and the Commonwealth Coat of Arms.
Inquiries about the licence and any use of this document should be emailed to copyright@dcceew.gov.au.

Cataloguing data
This publication (and any material sourced from it) should be attributed as: DCCEEW (2023) National Recovery Plan for the water mouse (Xeromys myoides). Department of Climate Change, Energy, the Environment and Water, Canberra. CC BY 4.0.
This publication is available at dcceew.gov.au/publications.
Department of Climate Change, Energy, the Environment and Water
GPO Box 3090 Canberra ACT 2601
Telephone 1800 803 772
Web dcceew.gov.au
Disclaimer
The Australian Government acting through the Department of Climate Change, Energy, the Environment and Water has exercised due care and skill in preparing and compiling the information and data in this publication. Notwithstanding, the Department of Climate Change, Energy, the Environment and Water, its employees and advisers disclaim all liability, including liability for negligence and for any loss, damage, injury, expense or cost incurred by any person as a result of accessing, using or relying on any of the information or data in this publication to the maximum extent permitted by law.
Acknowledgements
The Department of Climate Change, Energy, the Environment and Water recognises the ongoing connection of First Nations Peoples to culture and country across the water mouse distribution and beyond. We acknowledge First Nations Peoples as Traditional Owners, Custodians and Lore Keepers.
This recovery plan has been developed with the involvement and support of a broad range of partners, including Commonwealth and State environment departments, researchers, Aboriginal Corporations, Natural Resource Management bodies, local government, community group representatives, private land managers/custodians, and ecological consultants.
We would also like to thank all the partners who have assisted with recovery effort thus far, including the land managers and custodians who have actively contributed to recovery efforts on the land for which they are responsible.
The preparation of this plan was funded by the Commonwealth Government.
Image credits
Water Mouse (Xeromys myoides) at Sandringham Bay Conservation Park on the central Queensland Coast. John Atkinson via Wikimedia Commons: CC BY-SA 4.0.
Contents
Summary
1.1 Status
1.2 Description, habitat and distribution
1.3 Threats
1.4 Recovery plan vision, objectives and strategies
1.5 Criteria for success
1.6 Recovery team
2 Recovery information
2.1 Historical context
2.2 Conservation status
2.3 International obligations
2.4 Consultation
2.5 Partners
2.6 Species significance
3 Species information
3.1 Taxonomy
3.2 Description
3.3 Distribution
3.4 Habitat
3.5 Shelter and breeding
3.6 Diet and foraging
3.7 Movement
3.8 Connectivity and dispersal
3.9 Population size
3.10 Population trend
3.11 Important locations
3.12 Targeted survey methods
3.13 Intermittent detectability
4 Threats
4.1 Coastal development (urban and commercial)
4.2 Rapid sea level rise with climate change
4.3 Mangrove dieback
4.4 Prolonged and extreme inundation
4.5 Introduced predators
4.6 Cropping and aquaculture
4.7 Coastal pollution
4.8 Large herbivores
4.9 Fire
4.10 Weeds
4.11 Distribution of threats
4.12 Locations (populations) under pressure
4.13 Locations that are managed
4.14 Occurrence in protected areas
5 Recovery
5.1 Objectives
5.2 Actions
5.3 Priorities, timeframes and funding
5.4 Social and economic considerations
5.5 Ecological co-benefits
5.6 Plan implementation and evaluation
References
Expert Insight
Appendix A: Recorded water mouse locations
Appendix B: Priority locations for targeted water mouse detection surveys
Appendix C: Report framework for targeted water mouse surveys and incidental detections
Tables
Table 1: Affected interests and their relationship with the water mouse Recovery Plan
Table 2: Risk matrix
Table 3: Indicative summary of threat impacts across the water mouse distribution.
Table 4: Occurrence of water mouse in protected and heritage areas.
Table 5: Actions to ensure activities and developments in coastal areas within the modelled distribution of the water mouse are adequately assessed and regulated (Strategy 1).
Table 6: Actions to map water mouse habitat and locations at a fine scale (Strategy 2).
Table 7: Actions to develop clear and adaptive communications and implement tailored engagement processes (Strategy 3).
Table 8: Actions to support targeted water mouse detection surveys in areas of potential habitat (Strategy 4).
Table 9: Actions to support land managers and Custodians to include the water mouse in effective adaptive land management plans that identify and address local threats, and to implement these plans (Strategy 5).
Table 10: Actions to ensure effective water mouse population monitoring occurs (Strategy 6).
Table 11: Research questions to address knowledge gaps about water mouse ecology and detectability, and the impact of threats to the national population (Strategy 7).
Table 12: Priorities, actions, timeframes, estimated costs and primary funding sources for water mouse recovery.
Figures
Figure 1: A compilation of water mouse images displaying this species’ size and key features including grey fur with white flecking in some individuals, contrasting white belly, feet, and lower snout and cheeks, and short tail.
Figure 2: Examples of water mouse habitat along the southern Queensland coast: saltmarsh, coastal reeds, mangroves, mixed mangrove and saltmarsh, brackish sedgeland.
Figure 3: An example of water mouse habitat in the Mackay region.
Figure 4: Examples of mangroves at recorded water mouse locations in Cairns.
Figure 5: Water mouse habitat at Wando village on the Bensbach/Torassi River floodplain in southern New Guinea.
Figure 6: Examples of extensive mixed mangroves/saltmarsh, brackish and freshwater floodplain, and mangroves that may support undetected water mouse populations.
Figure 7: Examples of water mouse mud shelters at the hollow base of a tree.
Figure 8: Examples of water mouse mud shelters at the base of live and dead trees and in tree roots.
Figure 9: Example of a supralittoral bank (top left) and water mouse mud shelters in banks (top right, bottom).
Figure 10: Examples of water mouse mud shelters enhancing small, vegetated islands.
Figure 11: Examples of free-standing mud mound shelters in saltmarsh and mangrove communities along the southern Queensland coast.
Figure 12: An artificial mound colonised by water mouse.
Figure 13: Entry holes into water mouse mud shelters associated with hollow mangrove trees.
Figure 14: Water mouse mud shelter maintenance and repair: fresh mud tracks on top of a mound (top left), repaired damage to the side of a mound (top right), and mud spoil at the entry from internal maintenance (bottom).
Figure 15: Abundant crabs in mangroves that are occupied by the water mouse.
Figure 16: A water mouse moving through mangrove habitat.
Figure 17: Box trap setups for targeted water mouse detection (left) and research (right).
Figure 18: Water mouse detections on camera traps in southeast Queensalnd (top) and West Arnhem Land (bottom).
Figure 19: Typical feeding sign of a water mouse: crab claws and carapaces in neat piles (top and centre) or scattered near the entry of a mud shelter (bottom).
Figure 20: A water mouse on top of its mud shelter during a flood caused by an east coast low and high tide.
Figure 21: Camera trap detections of European red foxes disturbing and dismantling water mouse mounds along the southern Queensland coast.
Figure 22: Pig damage to water mouse habitat (left) and a mud mound shelter (right).
Figure 23: Significant cattle pugging and mud disturbance in water mouse habitat.
Figure 24: Burnt water mouse mud mound and surrounding habitat.
Maps
Map 1: Modelled water mouse distribution in Australia and southern New Guinea.
The purpose of this plan is to set out the management and research actions that are necessary to stabilise and better understand the national water mouse (Xeromys myoides) population over the next ten years.
International IUCN Red List of Threatened Species (2020): Vulnerable.
Commonwealth Environment Protection and Biodiversity Conservation Act 1999: Vulnerable.
Queensland Nature Conservation (Animals) Regulation 2020 under the Nature Conservation Act 1992: Vulnerable.
Northern Territory Territory Parks and Wildlife Conservation Act 2000: Data Deficient.
New South Wales and Western Australia: Not listed (may occur).
The water mouse is a small rodent with grey fur and a contrasting white belly, cheeks and upper lip. It is the only species in its genus and it occupies a unique ecological niche among rodents: primarily sheltering and breeding in permanent mud nests including in mangrove hollows above the high water line and feeding primarily on crabs and other marine invertebrates in the damp intertidal zone. Despite its name, the water mouse is a terrestrial species. It lives in intertidal mangrove and saltmarsh habitats, as well as coastal and subcoastal freshwater and brackish wetlands, swamps and floodplains.
Knowledge about the distribution and occurrence of the elusive water mouse is limited. It is a widespread species that is patchily recorded from three regions along the northern and eastern coastlines of Australia, and also from one location in southern New Guinea. It is most often encountered in coastal and island areas from the Coomera River to the Whitsunday coast in eastern Queensland. There are sporadic records along coastal areas of the Northern Territory, including the Tiwi Islands, and the far north Queensland coast from Cairns to the Hinchinbrook channel.
The water mouse is predicted to occur in additional unsurveyed areas of potential coastal and subcoastal habitat in Queensland, the Northern Territory, and New Guinea. It may also occur in unsurveyed potential habitat in Western Australia and New South Wales, beyond its current recorded range.
The primary threat to the survival of the water mouse is loss and fragmentation of habitat and adjacent areas due to coastal development, particularly along the central and southern Queensland coast. This will be exacerbated by sea-level rise causing coastal squeeze as climate change progresses. Coastal developments also increase the risk of water mouse habitat degradation from altered hydrology, exposure of acid sulfate soils, recreational activities (e.g. water vessel wash, quad bikes, four-wheel drives), excessive groundwater extraction, chemical leaching, and insecticide spray for mosquito control.
Other significant threats to the water mouse include damage to critical shelters and predation by European red fox and feral pig, mangrove dieback and saltwater intrusion, loss of cover and damage to critical shelters by large herbivores and fire, cropping and aquaculture, cat predation, and oil spill. As climate change progresses, the water mouse may also be threatened by prolonged inundation from severe cyclone and storm tidal surges and extreme seasonal flooding across its range. The risk level for these threats varies significantly across the water mouse distribution.
The long-term vision for the water mouse is that its distribution, population trends and threats are understood, and threats are effectively addressed to ensure the ongoing decline in the national population is stabilised and shows recovery despite anticipated future impacts of climate change. This water mouse recovery plan sets out objectives and actions that will ensure significant progress towards this goal over the 10-year life of the plan.
Recovery Plan objectives
- Significant impacts on the water mouse from coastal development and sea-level rise are effectively mitigated through sustainable development and habitat restoration initiatives.
- Current and potential future threats to water mouse are better understood and mitigated through research and adaptive management.
- The distribution and ecology of the water mouse is clarified, with effective management and monitoring actions implemented where it occurs. This includes areas primarily focused on conservation as well as locations with alternative primary objectives.
The following strategies are designed to meet these objectives within the 10-year lifespan of this recovery plan:
- Strategy 1: Ensure activities and developments in coastal areas within the current and future modelled water mouse distribution are adequately assessed, regulated, and managed to ensure no detrimental short-, medium-, or long-term impacts on the national population.
- Strategy 2: Map water mouse habitat and locations at a fine scale to ensure relevant land managers and Custodians are identified and engaged in water mouse recovery.
- Strategy 3: Develop clear and adaptive communications and implement tailored engagement processes to ensure relevant land managers and Custodians are effectively engaged in water mouse detection, management and monitoring.
- Strategy 4: Implement targeted water mouse detection surveys in areas of potential habitat across the water mouse distribution.
- At confirmed water mouse locations:
Strategy 5: Support current and future land managers and Custodians to include the water mouse in adaptive land management plans that support persistence and recovery by identifying local threats to this species, and implementing actions to address them.
Strategy 6: Ensure effective water mouse population monitoring occurs to enable local and national population trends, impacts of threats, and effectiveness of management actions to be assessed.
Strategy 7: Investigate water mouse ecology and detectability, and the impact of threats to the national population.
The recovery plan will be considered successful if by 2032:
- It can be demonstrated via population monitoring and approvals auditing that the water mouse population has not declined in abundance or occurrence due to coastal development, and
- Adaptive water mouse management plans are in place (or under development) and management actions are effectively implemented to address threats across the water mouse distribution, and
- Knowledge about water mouse ecology and the impacts of potential threats has increased and is incorporated into adaptive management plans, and
- Up-to-date water mouse information flows freely among partners due to effective facilitation by a Water Mouse Recovery Team, and
- Targeted survey effort to detect the water mouse has occurred at all priority locations across northern Australia (where safe and feasible to do so), and
- The ongoing decline in the national water mouse population is halted i.e. the population is demonstrated to be stable or recovering across its known distribution via an effective national monitoring program, and
- There is a significant increase in participation by Indigenous Peoples in cross-collaborative recovery planning and actione for the water mouse.
National Recovery Teams provide advice and assist in coordinating actions that are outlined in recovery plans. They include a diversity of representatives from organisations with responsibility for, and a direct interest in, the recovery of threatened species. A national Water Mouse Recovery Team is integral to successfully implementing the water mouse recovery plan across northern and eastern Australia, and one is to be established as a primary recovery action of this plan. A Water Mouse Recovery Team is necessary to collate, manage and disseminate information among a broad range of partners to ensure water mouse recovery efforts are collaborative and effective, and the vision of this plan is achieved within the next ten years.
This document constitutes the National Recovery Plan for the water mouse (Xeromys myoides) – an elusive rodent listed as Vulnerable under the Environment Protection and Biodiversity Conservation Act 1999 (EPBC Act).
This recovery plan is an update to the 2010 National Recovery Plan for the water mouse (DERM 2010). The 2010 plan was reviewed in April 2017 during a dedicated workshop in Brisbane to meet the requirements of Section 279 (2) of the EPBC Act. The review supported the development of a new recovery plan given the previous recovery plan had:
- Succeeded in guiding investment and actions for understanding and recovering the water mouse in southeast Queensland, which represented a significant component of the known and directly threatened water mouse national population at the time.
- Guided and ensured a strong interest in essential research that confirmed suspected threats (predation by European red fox and feral pig).
- Acted as a platform for vital policy development and mapping updates to better inform decision making for improved water mouse recovery outcomes.
The review confirmed with a high level of confidence that the national water mouse population was likely to be robust given its broad distribution and large extent of unsurveyed, undeveloped and likely habitat, but its conservation trajectory was considered to be deteriorating due to widespread threats from invasive mammals and sea-level changes, and localised declines from habitat loss due to urban and commercial development. There was a strong consensus that progress with recovery had been made in a small but important part of the water mouse range. However, it was also acknowledged that concerning threats were acting beyond this region and these required prioritising for the species as a whole.
The review recommended future recovery planning should prioritise actions to:
1) Minimise negative impacts to water mouse at locations along the central and southern Queensland coasts that are important for long-term species persistence.
2) Increase knowledge about the water mouse distribution across northern Australia.
The review concluded that effective and efficient use of available resources will be essential for implementing successful water mouse recovery actions over the next ten years.
Finally, the review recognised significant complexities in stopping the decline of the water mouse. Specifically, within the extensive distribution of the water mouse there is a wide range of partners and management capacities and also a broad diversity of land uses and development pressures. As such, a recovery plan is necessary to guide planning processes and adaptive management and monitoring programs to stabilise and better understand the national population of this poorly understood species.
The water mouse is listed as Vulnerable in the list of threatened species established under the Commonwealth Environment Protection and Biodiversity Conservation Act 1999 and the Queensland Nature Conservation (Animals) Regulation 2020 (under the Nature Conservation Act 1992). It is also listed as Vulnerable in The Action Plan for Australian Mammals 2012 (Woinarski et al 2014) and on the IUCN Red List of Threatened Species (Woinarski and Burbidge 2016). The water mouse is listed as Data Deficient under the Northern Territory Territory Parks and Wildlife Conservation Act 2000. It is yet to be detected in Western Australia or New South Wales and is not listed as threatened in these states.
Eligibility criteria were not included when the water mouse transferred to the EPBC Act threatened species list in July 2000 from the preceding Endangered Species Protection Act 1992. The IUCN listing criterion of B2ab(ii,iii,v) was based on it being plausible that the area of occupancy is less than 2000 km2 and it is a severely fragmented national population, combined with a continuing decline in area of occupancy, extent and quality of habitat, and number of mature individuals (Woinarski and Burbidge 2016).
Australia is a Signatory to the international Ramsar Convention (1971) to halt the worldwide loss of wetlands and to conserve, through wise use and management, those that remain. The water mouse is recorded from several Wetlands of International Importance that are listed under the Ramsar Convention (Australian Ramsar Wetlands 2021) and thus fall under international obligations for wise use and management to ensure they maintain their ecological character within the context of sustainable development (DAWE 2012):
1) Moreton Bay in southeast Queensland,
2) Great Sandy Strait on the southern Queensland coast, and
3) Kakadu National Park in the Northern Territory.
Other Ramsar Wetlands of International Importance that occur within the modelled distribution of the water mouse and may provide habitat include Shoalwater and Corio Bays (Qld), Bowling Green Bay (Qld), Cobourg Peninsula (NT), Ord River Floodplain (WA), and Roebuck Bay (WA).
These wetlands all contain category I (intertidal forested wetlands) and category H (intertidal marshes) wetlands that do or may provide habitat for the water mouse, i.e. mangroves, intertidal marshes, tidal freshwater swamp forests, and tidal brackish and freshwater marshes.
There are water mouse records from four World Heritage Areas in Australia (UNESCO 2021): Kakadu National Park, Wet Tropics of Queensland, K’gari (Fraser Island), and Great Barrier Reef. It also occurs in the proposed Great Sandy World Heritage Area (2010).
Australia is a Party to the United Nations Convention on Biological Diversity (1992) whose objectives are to conserve biological diversity and promote sustainable development. The water mouse occurs in areas where coastal development is expanding due to rapid human population growth. A sustainable development approach is required to meet the international obligations of this treaty.
In 2019 the water mouse was downgraded from Appendix I to Appendix II of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) because it is not traded, there is no suspected or demonstrable potential demand for trade, and future commercial trade is unlikely (CITES 2019).
This Recovery Plan was developed through consultation with a diversity of partners. The consultation process brought together contributions from species and land management experts and land Custodians from a variety of organisations and cultural backgrounds to outline the current status of knowledge and information gaps across the water mouse distribution, identify threats, and outline potential management options. The Australian Government Department of Climate Change, Energy, the Environment and Water (DCCEEW) has worked with all recovery partners who have demonstrated an interest in recovery of the water mouse to prepare this plan.
A draft of the National Recovery Plan for the water mouse was published online for an extended consultation period, from 4 February to 30 September 2022 during which time further consultation occurred with recovery partners and interested parties. Submissions were invited from all organisations, community groups, agencies and members of the public. Comments received, and additional insights provided, during the ongoing partner consultation period were considered and the content of this plan was updated accordingly prior to finalisation.
The water mouse has an expansive modelled distribution across terrestrial and marine areas (and their interface) with a broad array of international, national, state, and local interests. The tenures and management arrangements are variable, with multiple overlapping and adjacent management interests in most of the areas where the water mouse is known or likely to occur. As such, there are a significant number and diversity of partners that are relevant to implementing this national water mouse recovery plan. A high level of collaboration among these partners is required to ensure effective recovery actions are identified, developed, managed, and implemented for the recovery of this species. Partners may include, but are not limited to, those outlined in Table 1 below.
Due to its extensive distribution, implementation of the national recovery plan for the water mouse will require collaboration with many Indigenous groups who have Custodianship and management responsibility for lands on which the water mouse does or may occur; or who have a cultural connection to such lands. As such, consultation and implementation of the actions outlined in this plan must consider the role and interests of Indigenous Peoples. All recovery actions are to be undertaken in a manner that respects the cultural traditions of Indigenous Peoples throughout the known and modelled distribution of the water mouse.
This Recovery Plan ensures Traditional Owner interests in relation to the recovery of the water mouse are appropriately represented through Aboriginal Corporations, Ranger Groups, Land Councils, Indigenous community groups, and membership in the Water Mouse Recovery Team.
Table 1: Affected interests and their relationship with the water mouse Recovery Plan
Category | Interest Parties* | Responsibilities | Benefits/Impacts |
Australian Government | Environment department/s | Develop, coordinate, and evaluate the Recovery Plan. Ensure the plan is implemented in Commonwealth areas. Provide funding support. Ensure compliance with the plan. Co-ordinate review of the plan. | Greater ability to deliver on international and domestic obligations regarding biodiversity conservation by improving the water mouse status. Enhanced ability to communicate and exchange information among water mouse partners. |
| Indigenous affairs department/s | Provide funding and support for Indigenous Ranger Groups. | Support provided for implementation of programs to enhance connection and custodianship of Indigenous People and Country. |
| Education and research department/s, Australian Research Council | Provide funding support to address significant knowledge gaps. | Support provided for environment department to deliver on international and domestic obligations regarding biodiversity conservation through high quality, targeted research. |
| Development, planning, agriculture, fisheries, and defence departments | Comply with the plan. Submit compliant referrals for activities with significant impacts on the water mouse within its modelled distribution or where it may occur. | Enhanced ability to design and implement ecologically sustainable regional development models, and land management programs, on areas of responsibility. |
State and Territory Governments | Environment and land management departments: Qlda, NTb, WAb. | Assist to develop the plan. Ensure the plan is implemented in state and territory areas. Provide funding support. Provide progress updates. | Greater ability to deliver on state and territory obligations regarding biodiversity conservation and sustainable development. Enhanced ability to exchange water mouse information among partners. |
| Development, planning, agriculture, and fisheries departments: Qld, NT. | Comply with the plan. Submit compliant referrals for activities with significant impacts on the water mouse within its modelled distribution or where it may occur. | Increased knowledge about water mouse to support the design and implementation of ecologically sustainable regional development models. |
Aboriginal Corporations and Indigenous Ranger Groups | aQuandamooka Yoolooburrabee, Ngarang-Wal, Kabi Kabi First Nations Peoples, Bunya People, Butchulla, “Bailai, Gurang, Gooreng Gooreng, Taribelang Bunda”, Yuwi, Dawul Wuru, Gumay Walubara (Yidinji), Yirrganydji, Arafura Swamp Rangers, Wanga Djakamirr, Bawinanga, Djelk, Kakadu National Park Board, Warnbi, Tiwi, Malak Malak. bMooloolah Kabi Kabi Lands Council, Woppaburra People, Darumbal People, Koinmerburra, Kyburra Munda Yalga, Warga Badda Nywaigai, Mandubarra, Warrgamay Bada Bada, Girramay People, Gulngay Kinjufile, Djiru Warrangburra, Girrungun, Mamu, Gunggandji-Mandingalbay Yidinji, Gunggandji, Jabalbina Yalanji, Uku Baja Muliku, Hopevale Congress, Walmbaar, Dhubbi Warra, Balnggarrawarra, “Cape Melville, Flinders & Howick Islands”, Rinyirru (Lakefield), Yintjingga, Lama Lama, Uutaalnganu, Angkum, Kaapay Kuuyun, Kuuku Ya’u, Northern Kuuku Ya’u Kanthanampu, Bromley, Wuthathi, Ipima Ikaya, Apudthama, Kaurareg, Malu Lamar, Mualgal, Mura Badulgal, Goemulgaw, Maluilgal, Malu Ki’ai, Dauanalgaw, Saibai Mura Buway, Gebaralgal, Magani Lagaugal, Wakeyama, Warreberalgal, Porumalgal, Garboi, Masigalgal, Ugar Ged Kem Le Zeuber Er Kep Le, Erubam Le, Mer Gedkem Le, Seven Rivers, Mapoon, Mokwiri, Nanum Wungthim, Ngan Aak-Kunch, Pormparaaw, Abm Elgoring Ambung, Kowanyama, Gkuthaarn and Kukutj, Gulf Region, Gangalidda and Garawa, Yugul Mangi, Li-Anthawirriyarra, Numbulwar Numburindi Amalahgayag Inyung, Anindilyakwa, Yirrkala, Dhimurru, Laynhapuy, Gumurr Marthakal, Mardbalk, Adjumarllarl, Crocodile Island, Mardbalk, Garngi, Garig Gunak Barlu National Park Board, Kenbi, Belyuen, Larrakia Nation, Bulgul, Ngatpuk, Wudicupildiyerr, Thamarrurr, Miriuwung and Gajerrong, Balanggara, Wunambal Gaambera, Dambimangari, Mayala Inninalang, Warrwa People, Walalakoo, Gogolanyngor, Nimanburr, Nyul Nyul, Bardi and Jawi Niimidiman, Yawuru, Nyangumarta Karajarri/Karajarri Traditional Lands Association. | Assist to develop the plan. Implement the plan by creating and incorporating new knowledge into land management plans and actions for Healthy Country outcomes. Provide progress updates. | Enhanced ability for Indigenous Peoples to deliver on local obligations for Healthy Country Custodianship. Increased connection between Indigenous Peoples and Country. |
Indigenous Land Councils | aNorthern (NT). bCape York, North Queensland, Kimberley. | Facilitate partner connection. Facilitate and implement on-ground management and monitoring activities. | Increased connection between Indigenous Peoples and Country. Enhanced ability for Indigenous Peoples to deliver on local obligations for Healthy Country Custodianship. |
Natural Resource Management Bodies | aHealthy Land and Water, Burnett-Mary, Fitzroy Basin Association, Reef Catchments, Terrain, Territory. bNQ Dry Tropics, Cape York, Torres Strait Regional Authority, Northern Gulf Resource Management Group, Southern Gulf, Rangelands (WA). | Seek funding and provide support. Facilitate partner connection and regional plan integration. Facilitate and implement on-ground management and monitoring activities. Provide progress updates. | Increased connection between people and country. Increased capacity to inform land managers and owners about biodiversity values. Increased knowledge about water mouse to support and prioritise local and regional plans and actions. |
Local Councils | aGold Coast City, Redland City, Moreton Bay Regional, Sunshine Coast, Noosa Shire, Gympie Regional, Fraser Coast Regional, Bundaberg Regional, Gladstone Regional, Livingstone Regional, Mackay Regional, Whitsunday Regional, Cairns Regional; East Arnhem, West Arnhem, Tiwi Islands Regional, Tiwi Land, Coomalie. bBrisbane City, Isaac Regional, Burdekin Shire, Townsville City, Hinchinbrook Shire, Palm Island Aboriginal Shire, Cassowary Coast Regional, Yarrabah Aboriginal Shire, Douglas Shire, Hope Vale Aboriginal Shire, Cook Shire, Lockhart River Aboriginal Shire, Torres Shire, Torres Strait Island Regional, Northern Peninsula Area Regional, Mapoon Aboriginal Shire, Napranum Aboriginal Shire, Weipa Town Authority, Aurakun Shire, Pormpuraaw Aboriginal Shire, Kowanyama Aboriginal Shire, Carpentaria Shire, Mornington Shire, Burke Shire, Roper Gulf, Anindilyakwa Land, Litchfield, Darwin, West Daly, Victoria Daly, Wyndham-East Kimberley, Derby-West Kimberley, Broome. | Provide funding and support. Assist to develop the plan. Ensure the plan is implemented in local council areas. Comply with the plan. Submit compliant referrals for activities with significant impacts on the water mouse within its modelled distribution or where it may occur. Provide progress updates. | Greater ability to deliver on local obligations regarding biodiversity conservation and sustainable development. Increased knowledge about water mouse to support development and implementation of ecologically sustainable local development models and land management programs. Informed decision making regarding the EPBC Act referral and assessment process. |
Private Landholders | aTandora Grazing Pty Ltd, Bustard Bay graziers, Bush Heritage Australia. bMarra Land Trust, Seven Emu Station, Australian Wildlife Conservancy. | Implement the plan. Provide progress updates. | Enhanced ability to deliver on environmentally sustainable land management and biodiversity conservation obligations through threatened species management. |
Commercial Operators | Current and future developers within the modelled distribution (Map 1). Includes urban, commercial, airport, seaport, agriculture and aquaculture developments. | Comply with the plan. Submit compliant referrals for activities with significant impacts on the water mouse. | Informed decision making regarding the EPBC Act referral and assessment process. Enhanced ability to develop and implement ecologically sustainable urban and commercial (including agricultural and industrial) developments and activities. |
| Ecological Consultants/Ecologists | Implement the plan. Provide progress updates. | Contribute to knowledge about the distribution, habitat requirements and ecology of the water mouse for effective management. |
Researchers | Queensland Museum, Australian Museum, The Australian National University, Griffith University, The University of Queensland, The Queensland University of Technology, University of the Sunshine Coast, James Cook University, Charles Darwin University and others. | Address significant knowledge gaps. Report on progress. | Assist partners to meet obligations. |
Community Groups e.g. Coastcare, Landcare, Catchment Groups | aBribie Island Environmental Protection Association, Maroochy Waterwatch Inc., Sarina Landcare Catchment Management Association Inc., Pioneer Catchment and Landcare Group, Whitsunday Catchment Landcare, and others. bGamarrwa Nuwal Landcare, Landcare NT, and others. | Implement the plan. Provide progress updates. | Enhanced ability to deliver on local biodiversity conservation interests. |
Conservation Organisations | E.g. International Union for Conservation of Nature, World Wide Fund for Nature, The Mohamed bin Zayed Species Conservation Fund, MangroveWatch, Ian Potter Foundation, The Wilderness Society, Wildlife Preservation Society of Queensland, Queensland Conservation Council and others. | Provide funding. Facilitate partner connection. Facilitate information exchange and public communications. | Enhanced ability to deliver on biodiversity conservation obligations at various levels. |
The Public | People interacting with known and potential water mouse habitat and buffer zones for general living, recreation and tourism. | Report potential water mouse detections. Comply with the plan. | Enhanced ability to connect and engage with nature. |
* Interest parties are indicative.
a Parties with water mouse records on the land they hold or manage, or for which they are Custodians e.g. Traditional Owners, environment agencies.
b Parties with the potential for water mouse to occur on the land they hold or manage, or for which they are Custodians.
Scientific
The water mouse is the only extant species within the genus Xeromys (Wilson and Reeder 2005; Benfer et al. 2014). Its ecological niche – sheltering in constructed or modified mud shelters, primarily inhabiting tidal and brackish areas, and primarily feeding on marine invertebrates – appears to be unique among rodents globally (Van Dyck 1996; Van Dyck & Gynther 2003; Wilson & Reeder 2005).
Environmental
The water mouse is a coastal wetland health indicator due to its reliance on wetlands for all its life requirements, its sensitivity to disturbance, and its position near the top of the food chain (Ball et al. 2004; Van Dyck et al. 2006; DES 2013; WMRG 2022).
Over the last ten years, the water mouse has been a focus of Caring for Our Country, National Landcare, Reef Trust, Biodiversity Fund, and Fisheries Habitat Restoration programs in the Moreton Bay, Sunshine Coast, Great Sandy Strait (including K’Gari/Fraser Island), Burnett-Mary, Capricorn and Curtis, and Mackay and Whitsunday regions in Queensland, and also in the Northern Territory.
Indigenous
The water mouse does, or is predicted to, occur across important coastal Country for many Aboriginal and Torres Strait Island Peoples (Table 1). It is a recognised protected area value for the Djelk and Bawinanga, and Thamarrur Traditional Owners and the Arafura Swamp Rangers in the Northern Territory, and for the Quandamooka Yoolooburrabee and Bunya People Traditional Owners in southeast Queensland (Kaluza 2013; QYAC QPWS & P 2020).
In southeast Queensland, the water mouse is a culturally significant species for the Quandamooka People (HLW 2022) and the Kabi Kabi First Nations Peoples have custodial responsibilities for managing and protecting water mouse on Kabi Kabi Country (SCC 2022).
In east Arnhem Land (Northern Territory), the water mouse and Rakali/water rat (Hydromys chrysogaster) are differentiated from other rodents by the use of a single generic name for both species, which parallels their taxonomic relationship (Woinarski et al. 2000). In contrast, the water mouse is not differentiated from other small rodents by Indigenous villagers in Wando, New Guinea (Hitchcock 1998).
Social
The water mouse is poorly known across its distribution. However, it has significant social value in parts of its distribution where there is a history of custodianship. Groups with a strong social interest in the water mouse include land managers and Custodians and natural resource management and community groups along the southern Queensland coast and islands, and researchers and their affiliates in southeast Queensland and the Mackay region.
Economic
The water mouse is not traded (CITES 2019) and there are no recorded economic values for this species.
The depth of recorded information about the water mouse varies significantly with location: from detailed knowledge about habitats and habits along the southern Queensland coast to sporadic sightings across coastal areas of the Northern Territory, New Guinea, and far north Queensland. The information presented here focuses on aspects of water mouse ecology that are relevant to recovery objectives and actions and that highlight critical information gaps.
Uncontroversially accepted as Xeromys myoides Thomas (1889) (Rodentia: Muridae) and commonly called the water mouse. It is also recorded in the literature as the false water rat or false water-rat in reference to its closest relative in Australia the native Rakali/water rat (Hydromys chrysogaster). In some locations it is colloquially known as the mangrove mouse.
The water mouse is the only living member of the genus Xeromys. A genetic analysis of 120 species of rodents from the Hydromyini tribe in New Guinea and Australia by Roycroft et al. (2022) suggested the water mouse and the Rakali/water rat both evolved in New Guinea before independently colonising Australia. They are the only two species within the Hydromys division that occur in Australia, yet they are only distantly related to each other within this diverse division of at least 17 species. The closest relatives of the water mouse occur in New Guinea, being several species of moss mouse (Pseudohydromys spp.) and the northern groove-toothed shrew mouse (Microhydromys richardsoni).
Despite its disjunct distribution, a phylogeographic and population genetic analysis strongly supports the current designation of a single species and no subspecies for the water mouse in Australia (Benfer et al. 2014). The genetic relationship between the water mouse in Australia and southern New Guinea is unknown due to a lack of genetic material from New Guinea (Benfer et al. 2014), although it is morphologically indistinct (Hitchcock 1998).
The water mouse is a small and distinctive rodent that is about twice the size of a house mouse (Mus musculus) (Thomas 1889). It has a maximum recorded head-body length of 126 mm and a maximum weight of 64 g (Gynther & Janetzki 2013). The pelt, which is water- and mud-resistant, is silky and dark steel-grey in colour with a characteristic abrupt change to a pure white underbelly, lower snout and cheeks (Thomas 1889; Redhead & McKean 1975; Van Dyck 1996; Gynther & Janetzki 2013). There is usually white spotting through the fur of mature adults from Queensland (Van Dyck 1996), and the fur of older individuals can be grizzled grey with a rufous wash to the sides (Gynther & Janetzki 2013).
In the field, the water mouse can be distinguished from other rodents by its colour, silky fur, and tail that is shorter than its head-body length (Gynther & Janetzki 2013). Despite occurring in intertidal and semi-aquatic habitats, the hind feet of the water mouse are not webbed (Thomas 1889). The water mouse has a strong, acrid odour (Gynther & Janetzki 2013).
Figure 1: A compilation of water mouse images displaying this species’ size and key features including grey fur with white flecking in some individuals, contrasting white belly, feet, and lower snout and cheeks, and short tail.






Sources: © Ian Gynther (top left & top right @ Maroochy River in 2014; centre left @ Bribie Island; bottom left @ west K’gari/Fraser Island in 2016), © Alex Dudley (centre right), and © Wildwise Environmental (bottom right @ Sunshine Coast).
Recorded knowledge about the water mouse distribution is limited to three disparate locations in coastal and subcoastal northern Australia:
1) The southern and central Queensland coast (Coomera to Cannonvale).
2) Far north Queensland.
3) The Top End of the Northern Territory including the Tiwi Islands.
Outside Australia, the water mouse is known from the coastal floodplains of southern New Guinea (Hitchcock 1998).
The water mouse is rarely encountered, with records scattered throughout its range. It is known to occur in many coastal areas from Coomera on the Gold Coast to Cannonvale on the Whitsunday Coast. Extensive water mouse surveys and detections have occurred in southern Queensland (Dwyer et al. 1979; Van Dyck 1996; Burnham 2000; Van Dyck & Gynther 2003; Gynther 2011; Kaluza et al. 2016; Kaluza 2016a; 2016b; 2016c; 2016d; 2016e; 2016g; 2018; Sutherland 2017), and around Mackay (Ball et al. 2004). Sporadic detections have occurred in intervening areas (e.g. QGC 2013).
In far north Queensland, there are credible reports of water mouse in the Hinchinbrook Channel and the Mourilyan area in the late 1990s and early 2000s (WMRG 2022) and confirmed records from Cairns in 2017 (Ball & Mitchell 2018). Within the greater Cairns area it is known from the lower Barron River delta, with confirmed sightings at Barr Creek and adjacent to the airport (Ball & Mitchell 2018) and detection of feeding sign on Redden Island and at the mouth of Richter Creek (Mitchell 2021 pers. comm.). There are no recorded water mouse detections from coastal northern Australia between Cairns and Arnhem Land.
The water mouse is poorly known from a few dispersed records in the Northern Territory: the floodplains of the Glyde River and Tomkinson River in Arnhem Land, the South Alligator River in Kakadu National Park, Andranangoo Creek on Melville Island, and the Daly River (Redhead & McKean 1975; Magnusson et al. 1976; Woinarski 2000; Woinarski et al. 2000).
In southern New Guinea, the water mouse is recorded from Wando Village in the Tonda Wildlife Management Area (Hitchcock 1998; Hitchcock and Gabriel 2015). Wando Village is on a floodplain of the Bensbach/Torassi River at the northern extent of a vast lowland coastal floodplain that covers much of southern New Guinea including areas adjacent to the islands of the Torres Strait in far north Queensland (Paijmans et al. 1971; Hitchcock 2010).
The water mouse is predicted to occur in additional coastal and subcoastal areas of north Queensland, southern New Guinea and the Northern Territory (Magnusson et al. 1976; Ovington 1978; Dickman et al. 2000; Ball 2004). It is unlikely to be detected during general fauna survey programs (Ball et al. 2004) and most of its modelled distribution is in remote areas that can be challenging to access and survey (Van Dyck 1994; Ball 2004). Targeted surveys for water mouse in remote Australia over the last 20 years have been limited to a few of the historical detection locations in the Northern Territory (Woinarski et al. 2000; Low Choy & Fegan 2012; ASRAC 2017), opportunistic additions to general flora and fauna survey programs in the Torres Strait (Fell et al. 2018; Reis et al. 2018, 2020), a targeted survey near the Cairns airport following an incidental report (Ball & Mitchell 2018), and surveys for development approvals in Gladstone, Darwin Harbour and around Cairns. No targeted water mouse surveys are known to have occurred in Cape York, the Gulf of Carpentaria or New Guinea.
The western limit of the water mouse distribution is unclear. The most westerly records are from the Daly River area in the northwest Northern Territory (Redhead & McKean 1975). However, there are coastal areas with the potential to harbour water mouse habitat between the Daly River and the west Kimberley coast in Western Australia (Ovington 1978; Morris 2000). No targeted water mouse surveys are known to have occurred in Western Australia.
Unsuccessful targeted searches for water mouse have occurred along the northern New South Wales coast from the Richmond River at Ballina to just north of the Queensland border (Van Dyck & Gynther 2003). This suggests Coomera River and South Stradbroke Island in Queensland may be the southern limits of the water mouse distribution along the east Australian coast (Van Dyck & Gynther 2003; Adkins 2021 pers. comm.).
There are records of water mouse occurring in non-tidal areas up to 15 km inland in southern Queensland (Dwyer et al. 1979; Kaluza et al. 2016), 20-25 km inland in the Northern Territory (Redhead & McKean 1975; Magnusson et al. 1976) and 30 km inland in New Guinea (Hitchcock 1998; 2010).
In lieu of detailed knowledge about the occurrence of this elusive species, particularly in remote areas with potential habitat, the modelled distribution of the water mouse (i.e. known and likely to occur) is continuous along coastal areas of Queensland and the Northern Territory (Map 1). It may also occur along the Kimberley coast in Western Australia and in northern New South Wales.
Map 1: Modelled water mouse distribution in Australia and southern New Guinea.
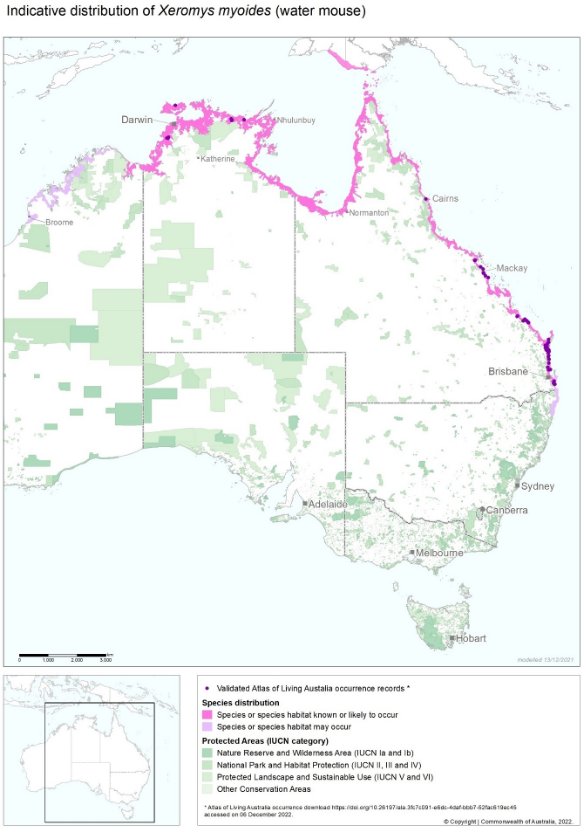
Source: © Commonwealth Department of Climate Change, Energy, the Environment and Water. Atlas of Living Australia (ALA) occurrence records download from ALA Xeromys myoides Occurrence Records on 13 December 2021. Note the ALA Public Records do not include the confirmed record in southern New Guinea or the reports of water mouse in the Hinichinbrook Channel of far north Queensland.
As a primarily intertidal species, the water mouse is considered to occur on both terrestrial and marine Country. It occurs within, and on the boundary of, several protected and recognised biodiversity areas including national and marine parks, Indigenous protected areas, exclusive Native Title areas, Ramsar listed wetlands, key biodiversity areas and nationally important wetlands of Australia. It also occurs on Department of Defence land, forestry reserves and private property. Recorded water mouse locations, land tenure and abundance information are outlined in Appendix A.
Knowledge about water mouse habitat requirements varies from well understood in southeast Queensland to partially known along the central Queensland coast to very limited across coastal areas of north Queensland, the Northern Territory, and southern New Guinea.
General habitat attributes
The water mouse is known to inhabit intertidal and supratidal areas, and subcoastal brackish to freshwater wetlands and floodplains up to 30 km inland. It is recorded from:
- Mangroves,
- Marine couch (Sporobolus virginicus) grasslands,
- Chenopod shrublands,
- Sedgelands,
- Reedy swamps,
- Melaleuca swamps,
- Seasonally inundated grassy floodplains, and
- Coastal wet heathlands.
The water mouse does not occur in urban developments or agricultural fields (Kaluza 2013).
Although the composition of water mouse habitat appears to vary across its distribution (see below), the following environmental attributes are required for an area to be occupied:
- Suitable – generally intact – hydrological flows (tidal and/or freshwater), and
- Ample food resources on a damp substrate (see Section 3.6), and
- Suitable mud substrate and/or scaffolding (e.g. mangrove roots or hollows, supralittoral bank) on – or slightly above – the high-water mark for permanent shelter construction or burrowing and maintenance (see Section 3.5), and
- Protected locations for shelters (e.g. protected from frequent submergence, erosive waves and/or water flows; trampling, rooting, dismantling; fire), and
- Vegetation cover between shelter and feeding grounds, or
- The capacity to develop these attributes in the future via targeted intervention (e.g. coastal remediation, invasive animal control, habitat supplementation) and/or natural processes (e.g. vegetation regeneration, hollow development).
Habitat information is sourced from McDougall (1944), Redhead & McKean (1975), Magnusson et al. (1976), Dwyer et al (1979), Van Dyck et al. (1992; 2013), Van Dyck (1994; 1996; 1997), Hitchcock (1998), Burnham (2000), Woinarski et al. (2000), Van Dyck & Gynther (2003), Ball (2004; 2013), Gynther & Janetzki (2008; 2013), Russell & Hale (2009), Gynther (2011), Kaluza (2013), Kaluza & Bolzenius (2015); Kaluza et al. (2016), Kaluza (2016b; 2016c; 2018; 2019) and Ball & Mitchell (2018).
A captive water mouse maintained its body weight with no access to free water for eight weeks (Van Dyck 1996), suggesting access to freshwater for drinking may not be a habitat requirement for this species. There is some indication that a neutral soil acidity value may be important (Kaluza 2021 pers. comm.).
In tidal areas, the water mouse requires a combination of easy access to the productive mangrove zone for food resources and protection from inundation and wave action for critical shelters (Van Dyck 1996; Van Dyck & Gynther 2003; Russel & Hale 2009; Kaluza 2018).
On-ground assessments are required to confirm areas of potential water mouse habitat across its modelled distribution (Map 1).
The water mouse is a widespread but elusive species. A clear understanding about its local habitat requirements is yet to be developed for most of its modelled distribution.
Specific habitat: southern Queensland coast (Gold Coast to Gladstone)
Water mouse habitat is well understood along the southern Queensland coast due to targeted detection surveys in the majority of areas that may support habitat (Van Dyck 1996; Burnham 2000; Van Dyck & Gynther 2003; Gynther 2011; Kaluza 2013; 2016a; 2016b; 2016c; 2016d; 2016e; 2016g; 2018).
Water mouse habitat in this region primarily consists of intertidal mangrove forests with adjacent saltmarsh communities (marine couch grasslands, sedgelands, reed beds and/or chenopod shrublands) with or without a wetland forest in the supratidal area. There are records from forests of grey mangrove (Avicenna marina), large-leafed orange mangrove (Bruguiera gymnorhiza), river mangrove (Aegiceras corniculatum), milky mangrove (Excoecaria agallocha), spotted mangrove (Rhizophora stylosa), spurred (or yellow) mangrove (Ceriops tagal), smooth-fruited yellow mangrove (Ceriops australis), broad-leaved paperbark (Melaleuca quinquenervia) and/or swamp she-oak (Casuarina glauca), and adjacent open vegetation communities (Van Dyck 1994, 1996; Van Dyck & Gynther 2003; Russel & Hale 2009; Gynther 2011; Kaluza & Bolzenius 2015; Kaluza 2016b; 2016c; 2013; 2018; 2019; Sutherland 2017).
There are also water mouse records from near-brackish and freshwater wetlands and swamps, and wet heath in southeast Queensland (Dwyer 1979; Russell & Hale 2009; Gynther 2011).
The water mouse is known to create a wide variety of protective mud shelter types in intertidal areas in this region, including within hollow mangroves and other trees and as distinctive free-standing mounds (see Section 3.5).
Figure 2: Examples of water mouse habitat along the southern Queensland coast: saltmarsh, coastal reeds, mangroves, mixed mangrove and saltmarsh, brackish sedgeland.







Sources: © Janina Kaluza (Top three panoramas @ Kauri Creek in 2015), © Ashley Rummell (large central image @ Maroochy River), © Melissa Bruton (bottom left & bottom centre @ Maroochy Wetlands Sanctuary in 2021) and © Ian Gynther (bottom right @ Bribie Island).
Recorded habitat: central Queensland coast (Gladstone to Cannonvale)
Due to limited survey effort, water mouse habitat requirements are not well defined along the central Queensland coast. This species may occur in coastal and subcoastal habitats that are yet to be recorded for this region. In the Gladstone-Curtis Island area water mouse detections have occurred in forests of spotted mangrove and yellow mangroves (Ceriops spp.) with adjacent marine couch (QGC 2013). Along the Mackay coast it occurs in forests of spurred mangrove and orange mangroves (Bruguiera spp.) (Ball 2004). The water mouse occurs patchily in mangrove forests along the Mackay coast where extensive targeted detection surveys have occurred, with the cause of this patchiness unclear (Ball 2004).
Freshwater areas that may provide habitat for the water mouse are now rare along the Mackay coast (Ball 2004; Ball 2021 pers. comm.). There is a historical record of five water mice collected from a permanent grassy Pandanus swamp about “one mile from the sea” (McDougall 1944).
In this region, the water mouse mostly uses mud ramp shelters constructed among the buttress roots of live and dead mangroves, and it may also construct tunnels into supralittoral banks (Ball 2004; Ball 2021 pers. comm.). The water mouse rarely creates distinctive freestanding mounds along the central Queensland coast (Ball 2021 pers. comm.). A suspected mud shelter in a human spoil pile is reported from Curtis Island (QGC 2013).
Figure 3: An example of water mouse habitat in the Mackay region.

Source: © Derek Ball.
Recorded habitat: far north Queensland
Recorded water mouse habitat information for far north Queensland is limited to two confirmed observations and the detection of feeding sign within tidal areas of the lower Barron River delta. Here, the water mouse has been recorded in mangrove forests dominated by spurred mangrove and/or smooth-fruited yellow mangrove with orange mangroves, and in backswamps (Ball & Mitchell 2018; Mitchell 2021 pers. comm.).
There is no recorded information about water mouse shelters in the Cairns region. Anecdotal reports suggest the presence of free-standing mounds in the Hinchinbrook channel (WMRG 2022).
Figure 4: Examples of mangroves at recorded water mouse locations in Cairns.


Source: © Melissa Bruton (left @ Jack Barnes Bicentennial Boardwalk in 2022; right @ Redden Island in 2022).
Recorded habitat: Northern Territory
The water mouse is recorded from widely dispersed locations across the Northern Territory. It occurs in both tidal and freshwater areas, with records from mangroves, saltmarsh and ephemeral freshwater wetlands (Redhead & McKean 1975; Magnusson et al. 1976; Woinarski et al. 2000). Detailed information is provided below for each of the widely dispersed detection locations.
Arnhem Land (Glyde River): Extensive (> 30 km2) seasonally inundated marine couch and nutgrass (Cyperus scariosus) grassland on a seasonal floodplain. Scattered low chenier ridges and patches of low chenopod shrubland punctuate the grassland, which has abundant crab activity in the dry season (Woinarski et al. 2000).
Arnhem Land (Tomkinson River): A tiny (4 m x 4 m) patch of marine couch surrounded by tidal mangroves: grey mangrove, white-flowered black mangrove (Lumnitzera racemosa), milky mangrove and spurred mangrove (Magnusson et al. 1976). A water mouse was also recovered from the stomach of a crocodile much further upstream along the same river, in an area with black ebony (Diospyros humilis), grey mangrove, river mangrove, large bluegrass (Ischaemum australe), marine couch and nutgrass (Magnusson et al. 1976).
Kakadu National Park: The coastal plain and tidal section of the South Alligator River (Parker 1973; Woinarski 2004). Detailed habitat information is not available for the single historical record from 1903.
Melville Island: Tall closed forest of small-flowered orange mangrove (Bruguiera parviflora) and spurred mangrove (Magnusson et al. 1976).
Daly River: Receding grassy ephemeral freshwater lagoons surrounded by Melaleuca sp. and freshwater mangrove (Barringtonia acutangula) with a good cover of introduced para grass (Urochloa mutica) (Redhead & McKean 1975).
A sizeable mud mound shelter housing three water mice was found interlocked within the buttress of a small-flowered orange mangrove tree on Melville Island off the Northern Territory coast (Magnusson et al. 1976). This is the only confirmed water mouse shelter from along the Australia and New Guinea coastlines north of Cannonvale (near Mackay).
Recorded habitat: New Guinea (near Torres Strait)
The New Guinea detections were both at the same location, within 20 m of a seasonally inundated sedge-grass swamp dominated by Eleocharis spp. and melaleuca, and within 100 m of swamp grassland floodplains of the Bensbach/Torassi River (Hitchcock 1998). The river meanders from this point through the extensive coastal plain to reach the coast. The coastal plain is comparable to the low-lying floodplains of Kakadu National Park: it is inundated in the wet season and consists of mainly sedge-grasslands with scattered Pandanus, and permanent and seasonal swamps with reeds and tall sedges, swamp grassland, and Melaleuca swamp forest, i.e. reported water mouse habitat elsewhere. The tidal flats fringing the coastal plain harbour mangrove forests (Paijmans et al. 1971; Hitchcock 1998; Hitchcock 2010).
There is no recorded information about water mouse shelters in New Guinea.
Figure 5: Water mouse habitat at Wando village on the Bensbach/Torassi River floodplain in southern New Guinea.


Source: © Garrick Hitchcock (left @ Wando village detection site in 1997; right @ adjacent swamp in 1995).
Areas with potential habitat
The majority of the modelled water mouse distribution contains remote unsurveyed areas with the potential to be habitat for this species. These areas are expansive and linearly distributed along the northern coastline from Mackay to the west Kimberley. There is currently insufficient knowledge about water mouse distribution and ecology in northern Australia to infer how much of this area is likely to be occupied.
Figure 6: Examples of extensive mixed mangroves/saltmarsh, brackish and freshwater floodplain, and mangroves that may support undetected water mouse populations.



Source: © Melissa Bruton (top left @ Illeda/Walcott Inlet WA in 2020; top right @ East Alligator River NT in 2013; bottom @ Boigu Qld in 2022).
Areas with water mouse habitat attributes that occur within the modelled distribution of the water mouse that have been surveyed without confirmed detections may be habitat that is temporarily unoccupied (see Section 3.12). A greater understanding about water mouse patterns of occurrence, response to prolonged inundation and other dynamic perturbations, population dynamics and dispersal, and detectability is required before these areas can be classed as unoccupied or unsuitable.
Areas supporting recovery
Unobstructed areas that are landward of occupied and potential water mouse habitat may be critical for sustaining and supporting the recovery of the national water mouse population in the future as sea levels rise and coastal ecosystems migrate inland (see Section 4.2).
The water mouse is known to decline in areas adjacent to development (Section 4.1). A development-free buffer zone of at least 200 m is required around water mouse habitat at locations under pressure (Section 4.12) to mitigate against declines. A larger buffer zone is required on the landward side in locations that are predicted to be under pressure in the future (Section 5.2.2) as sea levels rise and coastal habitats migrate inland with climate change (Traill et al. 2011). The required buffer distance will depend on terrain and sea-level rise predictions; it could be several kilometres on flat coastal plains.
Population connectivity is important for water mouse persistence, with genetic resilience known to have declined in at least one isolated location (Benfer et al. 2014). Intact coastal areas, with one or more of the environmental attributes that are required for an area to be occupied (Section 3.4), which are not currently occupied, or that have very low water mouse detectability (e.g. Laird Point on Curtis Island: ConocoPhillips 2020) may support dispersing individuals (Section 3.8) and be important for maintaining connectivity and genetic resilience at locations under pressure. These important areas are likely to:
- Occur between areas of known or potential habitat,
- Provide vegetation cover (Section 4.5) and temporary shelter resources (Section 3.5) to protect dispersing individuals from predation (Van Dyck 1996), and
- Provide sufficient food resources (Section 3.6) to sustain the high metabolic requirements of the water mouse (Van Dyck 1996) during dispersal.
Tropical locations recently impacted by a significant natural perturbation (mangrove dieback, storm surge, extreme wet season flood – see Sections 4.3 and 4.4) may be important for sustaining, and supporting the stabilisation of the national water mouse population in the future as these locations recover and water mice from adjacent or nearby refuge habitats re-colonise. Due to the dynamic nature of natural perturbations in tropical regions, adjacent refuge areas may also be impacted at a future time and the original impacted location may then become the refuge population. This highlights how variable the occurrence of water mouse populations may be in tropical locations and emphasises the potential importance of impacted areas as future refuge locations to support the stabilisation and recovery of this species.
Detrimental actions in areas supporting recovery are likely to interfere with the long-term persistence of the water mouse.
In tidal areas, the water mouse constructs or excavates a long-term multi-generational mud or peat structure for shelter and rest, for breeding, and for protection from predation. This long-term shelter – which can be a tunnel system in a bank, a mud-packed hollow tree or stump, or a complex structure often referred to as a ‘nest’ or ‘nest mound’ – is a critical and stable resource for the water mouse.
The water mouse can use a variety of natural environmental features as scaffolding to create or stabilise mud shelters including:
- A supralittoral bank (Van Dyck & Durbidge 1992; Van Dyck 1996; Van Dyck & Gynther 2003; Gynther 2011; Kaluza 2013; Kaluza 2018),
- Hollow trunks and limbs of grey mangrove (Van Dyck & Gynther 2003; Gynther 2011; Kaluza 2013),
- Hollow trunks of broad-leaved paperbark or swamp she-oak (Van Dyck & Gynther 2003),
- Hollow dead tree trunks, stumps, or limbs (Van Dyck & Gynther 2003; Gynther 2011),
- The base of live trees or shrubs (Van Dyck & Durbidge 1992; Kaluza 2013),
- Slender roots and trunks of mangroves (Kaluza 2013),
- Buttress roots of spurred mangrove, small-flowered orange mangrove or large-leafed orange mangrove (Magnusson et al. 1976; Ball et al. 2004),
- Underground roots of grey mangrove or river mangrove (Van Dyck & Gynther 2003),
- Underground roots of broad-leaved paperbark, swamp she-oak, or groundsel bush (Baccharis halimifolia) (Van Dyck & Gynther 2003; Gynther 2011; Kaluza 2013).
Figure 7: Examples of water mouse mud shelters at the hollow base of a tree.





Sources: © Ian Gynther (top left @ Kauri Creek; bottom left & bottom right @ Maroochy River in 2011), © Janina Kaluza (top centre @ Eurimbulla National Park in 2016) and © Melissa Bruton (top right @ Maroochy Wetlands Sanctuary in 2022).
Figure 8: Examples of water mouse mud shelters at the base of live and dead trees and in tree roots.





Sources: © Steve Van Dyck via Ian Gynther (top left) and © Ashley Rummell (all other images @ Maroochy River).
Figure 9: Example of a supralittoral bank (top left) and water mouse mud shelters in banks (top right, bottom).



Sources: © Melissa Bruton (top left @ Maroochy Wetlands Sanctuary), © Ashley Rummell (top right @ Maroochy Wetlands Sanctuary) and © Ian Gynther (bottom @ Bribie Island).
Figure 10: Examples of water mouse mud shelters enhancing small, vegetated islands.

 Sources: © Ian Gynther (top @ Donnybrook in the Pumicestone Passage) and © Ashley Rummell (bottom @ Coolum Creek Environment Reserve).
Sources: © Ian Gynther (top @ Donnybrook in the Pumicestone Passage) and © Ashley Rummell (bottom @ Coolum Creek Environment Reserve).
The water mouse can also create a free‑standing mud mound shelter best described as a ‘low soggy termite mound’, usually with a thick cover of ground vegetation (Van Dyck & Durbidge 1992; Van Dyck & Gynther 2003; Kaluza 2013). These structures are usually stabilised and/or covered by marine couch grass, reeds, sedges, and/or mangrove pneumatophores (Van Dyck & Durbidge 1992; Van Dyck & Gynther 2003; Kaluza 2013; Kaluza 2018). Free-standing water mouse mound shelters are common in southern Queensland (Burnham 2000; Van Dyck 1996; Van Dyck & Gynther 2003; Kaluza et al. 2016; Kaluza 2018), rare along the central Queensland coast (Ball 2004; Ball 2021 pers. comm.) and have been anecdotally reported from the Hinchinbrook Channel (WMRG 2022). They have not been reported from elsewhere within the water mouse distribution i.e. Cairns, and coastal areas and islands of the Northern Territory.
Figure 11: Examples of free-standing mud mound shelters in saltmarsh and mangrove communities along the southern Queensland coast.








Sources: © Ian Gynther (top left @ Noosa North Shore; top right @ Meldale in the Pumicestone Passage in 2022), © Ashley Rummell (middle top left & middle bottom right @ Maroochy River), © Raymond Donald via Janina Kaluza (middle top right in 2012), and © Janina Kaluza (bottom left @ Kauri Creek in 2015; bottom right @ Maroochy River in 2012).
The water mouse can opportunistically modify artificial structures such as bunds, spoil piles and artificially constructed mounds for shelter sites within otherwise undisturbed habitat (Van Dyck & Gynther 2003; Van Dyck et al. 2003). It can also use simplified tree shelters, hollow mangroves, ground debris and crab holes as temporary shelter sites (Magnusson et al. 1976; Van Dyck 1996; Van Dyck & Gynther 2003; Ball 2004).
Figure 12: An artificial mound colonised by water mouse.

Source: © Steve Van Dyck via Ian Gynther (Coomera River).
Additional photographs of water mouse shelters are available in Burnham (2000) and Van Dyck & Gynther (2003). Mud mounding crabs can create similar mud shelters to the water mouse, but they lack entrance holes and mud-formed tracks or runways (Burnham 2000).
Permanent shelters contain several internal chambers that are accessed via tunnels leading from elliptical entry holes; these are smooth around the margin and often linked by tracks of fresh mud-daubing on the outside of the shelter (Van Dyck & Gynther 2003; Kaluza 2019; Gynther 2021 pers. comm.). Tunnels within mounds can extend as far as 0.9 m below ground level (Magnusson et al. 1976; Van Dyck & Gynther 2003) and 20 m horizontally within earthen banks (Van Dyck 1996).
Figure 13: Entry holes into water mouse mud shelters associated with hollow mangrove trees.


Source: © Ian Gynther (left @ Poona in the Great Sandy Strait in 2012; right @ Maroochy River in 2011).
The location and type of permanent shelter structure that is constructed depends on the availability of environmental features, wave action and tidal range, adjacency to the mangrove zone with its highly productive food resources, and available water-free foraging period in tidal areas (Van Dyck 1996; Van Dyck & Gynther 2003, Kaluza 2018). Shelters along the southern Queensland coast usually occur on or just above the highest tide mark (Van Dyck & Durbidge 1992; Van Dyck & Gynther 2003; Russell & Hale 2009; Kaluza 2013; 2018) or within the regularly flooded mangrove zone where hollow trees enable protection from inundation (Ian Gynther 2021 pers. comm.). Confirmed water mouse shelters along the Mackay coast are all within the mangrove zone (Ball 2004). In the Great Sandy Strait, shelters are generally not located on open saltpans dominated by beaded samphire (Sarcocornia quinqueflora) or where wide expanses of saltpan occur between supralittoral and mangrove communities (Burnham 2000).
Constructed shelters require constant attention using mud-daubing – mud carried and manipulated in the mouth – to enlarge them and to repair water erosion and other damage (Van Dyck 1996; Van Dyck & Gynther 2003; Ball 2004; Gynther 2011; Kaluza 2013; Kaluza 2019).
Figure 14: Water mouse mud shelter maintenance and repair: fresh mud tracks on top of a mound (top left), repaired damage to the side of a mound (top right), and mud spoil at the entry from internal maintenance (bottom).




Sources: © Ian Gynther (top left @ Donnybrook in the Pumicestone Passage; bottom left @ west K’gari/Fraser Island in 2016; bottom right @ Bribie Island in 2009) and © Steve Van Dyck via Ian Gynther (top right @ Bribie Island).
Endoscope probing into the nest chambers of permanent shelters at Myora on Minjerribah/North Stradbroke Island recorded up to eight individuals of all ages and sexes, but with no more than one adult male (Van Dyck 1996). An average of two occupants was recorded in permanent shelters monitored with cameras along the Maroochy River (Kaluza et al. 2016).
There is no recorded information about water mouse shelters in freshwater habitats where large, complex structures may not be required due to an absence of daily fluctuations in water height (Gynther 2011). In these areas, the water mouse may use simple tunnels in banks around the margins of wetlands, or surface debris, for shelter and nest sites (Gynther et al. 2011).
Male and female water mice can be detected in breeding condition throughout most of the year in southeast Queensland and camera monitoring of shelters suggests they breed up to twice per year (Van Dyck 1996; Kaluza 2021 pers. comm.). A nest of four hairless young was detected in a shelter on Minjerribah/North Stradbroke Island (Van Dyck 1996).
The water mouse feeds primarily on sesarmid and grapsid crabs across its distribution (Magnusson 1976; Van Dyck 1996; Burnham 2000; Ball 2004; Ball & Mitchell 2018). In southeast Queensland, where it has been studied intensively, the water mouse also feeds on other crustaceans, as well as marine pulmonates, bivalves and polyclad flatworms within intertidal systems (Van Dyck 1996). It is likely that the water mouse has a diverse carnivorous diet across its range. It is reluctant to consume vegetables in captivity (Magnusson et al. 1976) and gut and scat analyses support a carnivorous diet (Van Dyck 1996). There is speculation that a high density of crab prey may be required for water mouse to occur and persist along the central Queensland coast (Ball 2004).
The water mouse is only known to forage at night and when the tide does not cover intertidal feeding areas (Van Dyck 1994). Food items are consumed under cover in sheltered locations (Van Dyck 1996, Ball 2004). Nocturnal foraging is likely to be unconstrained in areas without tidal influence such as the subcoastal lagoons, floodplains, and freshwater wetlands (Gynther 2011).
Low trap rates and a lack of feeding activity by radio-tracked water mice in freshwater habitat (Van Dyck 1996) suggest they may only forage in these areas when resources elsewhere are in short supply such as during flooding events (Gynther 2011). The diet, shelter and activity patterns of water mouse in freshwater environments are not known (Gynther 2011), although freshwater crabs may be a significant prey item on the extensive floodplains of the Northern Territory (Woinarski et al. 2000).
Figure 15: Abundant crabs in mangroves that are occupied by the water mouse.


Source: © Melissa Bruton (Cairns in 2022).
Recorded minimum water mouse home range sizes on Minjerribah/North Stradbroke Island varied from 0.3‑0.9 ha, with one outlier value of 3.4 ha at a site with widely spaced mature mangrove trees and very low substrate complexity (Van Dyck 1996). Most of the time was spent in non-overlapping 0.2 ha core areas (Van Dyck 1996). Agonistic encounters between individuals have been observed (Van Dyck & Gynther 2003).
Individuals emerge from shelters and follow the receding tide through sedgelands and mangroves before retreating as the tide – or daylight – returns (Van Dyck 1996). Radio-tracked individuals spent an average of 85 % of their time outside shelters in frenetic activity (Van Dyck 1996). In addition to feeding, individuals visit neighbouring shelters and favoured haunts, as well as participating in shelter maintenance (Van Dyck 1996; Kaluza et al. 2016).
The water mouse appears to have limited ability or propensity to swim (Thomas 1889; Magnusson et al. 1976; Van Dyck 1996) despite its name and semi water-adapted morphology (Redhead & McKean 1975).
The agility of the water mouse may vary, with individuals described as having limited ability to climb mangrove trees in southeast Queensland (Van Dyck 1994, 1996) yet ‘climbing agilely among the outer branches in a manner reminiscent of the pigmy [sic] possum’ in Arnhem Land (Magnusson et al. 1976).
Figure 16: A water mouse moving through mangrove habitat.

Source: © Ian Gynther (west K’gari/Fraser Island in 2016).
The water mouse has low genetic diversity across its distribution, and individuals from across its range are morphologically consistent, suggesting a recent radiation and significant historical population connectivity. There is some genetic variation across the national population, which is expected given its linear coastal distribution. Currently it is unclear if discrete boundaries in genetic structure among geographic regions are due to reduced water mouse gene flow among widely dispersed sampled locations (e.g. Mackay vs. Agnes Water) or an absence of genetic samples in intervening locations (Benfer et al. 2011).
There is currently no recorded information about the capacity for the water mouse to disperse, or about coastal barriers to water mouse population connectivity. The water mouse can travel over 600 m per night between shelter and feeding grounds (Van Dyck 1996), 1 km in a few hours (Kaluza 2021 pers. comm.), and 2.9 km overnight when foraging (Van Dyck 1994), and only small amounts of migration are required to maintain the genetic resilience of water mouse populations across its distribution (Benfer et al. 2014). This suggests that irregular dispersal events across large distances of seemingly inhospitable terrain may be sufficient to ensure connectivity and may explain the lack of significant genetic variation across the broad water mouse range. The contribution of flood events, currents, tides and cyclones to water mouse dispersal and genetic connectivity are unknown, as are the impacts of fragmentation caused by extensive coastal development in southeast Queensland.
The water mouse is one nationally important population with no significant genetic divergences across its broad distribution (Benfer et al. 2014). The most recent national population estimate is 10 000 mature individuals, although this figure has low reliability (Woinarski et al. 2014). Nationally, the water mouse is elusive, rare, and scattered (Gynther & Janetzki 2008) making it challenging to determine a confident estimate of the national and global population.
Locations with a high recorded water mouse abundance or density include:
- The west coast of Minjerribah/North Stradbroke Island in southeast Queensland where historical trap rates were relatively high at 8–24 % (Van Dyck 1996).
- The Pumicestone Passage-Bribie Island region in southeast Queensland, where there is a relatively high density of active shelters across a large area (Gynther 2011; Kaluza 2013; 2016b).
- The Maroochy River in southeast Queensland, where approximately 340–500 individuals are estimated to occur in a relatively small area (Kaluza et al. 2016).
- The Great Sandy Strait and K’Gari/Fraser Island region along the southern Queensland coast, where there is a relatively high abundance of active shelters across a broad area (Burnham 2000; Kaluza 2018). Within this region, Kauri Creek and Tin Can Inlet have particularly high abundances (Burnham 2000).
- Freshwater Point East on the central Queensland coast near Mackay, where ten individuals were trapped over 40 trap-nights for a very high relative trap rate of 25 % (Ball 2004).
Water Mouse abundance or density estimates are unavailable for north Queensland, the Northern Territory, and southern New Guinea.
There is no national population monitoring program for the water mouse and there are few ongoing standardised trend monitoring programs for this species across its distribution. Inference, rather than empirical assessment, has been used to assess the global water mouse population as declining (Woinarski & Burbidge 2016; CITES 2019).
The abundance of active water mouse shelters has been monitored annually since 2019 in the Maroochy Wetlands Sanctuary on the Sunshine Coast, and also at McCoys Creek on the Gold Coast. Population trend estimates are anticipated for these locations in the near future.
The water mouse and its shelters appear to have contracted from saltmarsh habitat in the Coomera River system (Van Dyck et al. 2006) and are now only known to occur in areas with large hollow-bearing mangroves (Adkins 2021, 2022 pers. comm.), and the number of active shelters appears to have declined at Hussey Creek in the Pumicestone Passage and near Camp Kerr in the Great Sandy Strait (Kaluza 2016b; 2018).
Active water mouse shelter sites have been censused in multiple locations across southeast Queensland and north to the Fraser Coast (Van Dyck 1996; Burnham 2000; Van Dyck & Gynther 2003; Gynther 2011; Kaluza 2013; 2016a; 2016c; 2016d; 2016e; 2016f; 2016h; 2018; Kaluza et al. 2016), and historical water mouse live trap rates are available for Minjerribah/North Stradbroke Island (Van Dyck 1996), Bribie Island (Gynther 2011) and along the Mackay coast (Ball et al. 2004). Standardised re-survey of these areas combined with a robust estimate for the number of mature individuals using each shelter is required to provide empirical information about water mouse population trends.
Water mouse population trends are unknown for the central Queensland coast, Cairns, across the Northern Territory and in southern New Guinea (Woinarski & Burbidge 2016).
Population monitoring using standardised repeatable methods is urgently required at water mouse locations to confidently assess the national population size, local trends, and relative threat impacts.
Locations with the greatest likelihood of sustaining a resilient water mouse population long-term despite climate change impacts:
- Will occur across broad geographic areas and retain connectivity with the greater national population as sea levels rise and coastal habitats migrate inland,
- Have limited exposure to, or have the capacity to adapt and recover from, periodic natural perturbations (e.g. storm surges, prolonged wet season inundation, mangrove dieback), and
- Have few threats (e.g. undeveloped and predator-free islands) or are managed by well-funded and co-ordinated land management and Custodianship groups who are able to implement effective on-ground actions to alleviate local threats.
Outcomes from targeted detection survey, population monitoring, genetic analysis, future habitat mapping, and adaptive management plan programs outlined in this recovery plan will enable these important locations to be identified.
The water mouse is a cryptic species that can be challenging to survey and detect. It is unlikely to be detected by most standard survey techniques (Ball 2004; but see Woinarski et al. 2000), requiring a targeted approach. Suitable water mouse survey techniques are:
1) Feeding sign and prey search. The water mouse leaves a distinctive feeding sign of intact crab carapaces and chewed claws (Figure 19) in a neat cumulative midden area in open or protected locations within the intertidal foraging zone, and on shelters (Van Dyck 1996; Ball et al. 2004; Ball & Mitchell 2018; Kaluza 2019).
2) Shelter site search. See section 3.5 for shelter details. The strong, acrid odour of the water mouse can be detected emanating from active shelter entries (Van Dyck & Gynther 2003; Kaluza 2013; Ball 2021 pers. comm.) and there is usually fresh mud and binding material – peat, dry leaves, sedges, crab shells – plastered on top (Van Dyck & Durbidge 1992). Shelters aren’t always detectable: the water mouse is known to occur in locations across the Northern Territory and Queensland where shelters are yet to be observed or confirmed (Magnusson et al. 1976; Dwyer et al. 1979; Woinarski et al. 2000; Ball 2004; Gynther 2011; Ball and Mitchell 2018).
3) Camera survey. The slate-grey colour of the water mouse, and the pure white belly and lower lip, are diagnostic characters that can be discerned on white-flash camera images for a confident identification (Ball & Mitchell 2018). The tail of the water mouse is short in comparison to other rodents, which enables it to be confirmed on greyscale camera images in some locations (Ball 2021 pers. comm.).
4) Box trap survey. Size A Elliott box traps at potential shelters and feeding stations (Van Dyck 1996; Kaluza et al. 2016; Ball & Mitchell 2018) and placed systematically throughout suitable habitat (Dwyer et al. 1979; Van Dyck 1996; Gynther 2011) have captured the water mouse.
Cameras and box traps have reliably detected water mouse when baited with pilchards, mullet, or gar (Van Dyck 1994; 1996; Ball 2004; Gynther 2011; Ball & Mitchell 2018).
Figure 17: Box trap setups for targeted water mouse detection (left) and research (right).


Source: © Ian Gynther (left @ Bribie Island; right @ Maroochy River in 2014).
Remote cameras are effective for detecting the water mouse, monitoring behaviour, and assessing presence-absence trends at large and small scales (Gillespie et al. 2015; Ball & Mitchell 2018; Kaluza 2021 pers. comm.). However, cameras are not suitable for monitoring population abundance trends for species where individuals are unable to be identified from images (Gillespie et al. 2015), as is the case for the water mouse.
Cameras may prove vital for confirming and monitoring water mouse occurrence in remote areas where shelter structures are unknown and/or the deployment of live-capture box traps is problematic. In-depth knowledge about expected tidal reach is required to safely deploy cameras and live traps (Van Dyck 1996).
Figure 18: Water mouse detections on camera traps in southeast Queensalnd (top) and West Arnhem Land (bottom).


Source: © Wildwise Environmental (top in 2021) and Indigenous Rangers (bottom in 2022).
Figure 19: Typical feeding sign of a water mouse: crab claws and carapaces in neat piles (top and centre) or scattered near the entry of a mud shelter (bottom).





Sources: © Janina Kaluza (top left @ Hussey Creek in 2012), © Derek Ball (top right @ Mackay region), © Ashley Rummell (centre left & centre right @ Maroochy River) and © Graham Webb (Maroochy Wetlands Sanctuary).
The use of conservation detection dogs for finding rare and cryptic species that occur at low densities or with clustered populations – such as the water mouse – is an emerging technique that is gaining traction due to it being an accurate, and time and cost-effective method (Reindl-Thompson et al. 2006; Long et al. 2007; Duggan et al. 2011; Leigh & Dominick 2015; Thomas et al. 2020). The cost-effectiveness of using conservation detection dogs has been proven in multiple assessments, due to the reduced effort that is necessary in the field to achieve a high to very high probability of detection (Harrison 2006; Long et al. 2007; Duggan et al. 2011; Chambers et al. 2015; Clare et al. 2015; Thomas et al. 2020). For the water mouse, which has a strong acrid odour (Gynther & Janetszki 2013), the training and use of detection dogs may be an effective method for finding shelter and feeding areas, particularly in the north of its range, where there is very little information about its occurrence. Collection of biological material (nest mud, scat, fur etc.) from easy to access locations in southeast Queensland (e.g. Gold Coast, Sunshine Coast, Mackay) could provide the olfactory cues that are necessary to train detection dogs for this species.
Pitfall traps set in dry floodplains in Arnhem Land have captured water mice (Woinarski et al. 2000) and two individuals became trapped in a freshly excavated latrine pit in New Guinea (Hitchcock 1998). Concurrent box trap surveys failed to detect the water mouse at the Arnhem Land site or in nearby mangrove, saline floodplain or freshwater swamp sites (Woinarski et al. 2000). As such, pitfall traps may be a useful – and potentially highly effective – method for surveying water mouse where environmental conditions ensure animal safety is not compromised.
It is possible to spotlight the water mouse foraging in open grey mangrove forests, where it can be positively identified by the distinctive white belly and dull mauve eye shine (Van Dyck 1996). However, spotlighting is generally not a cost-effective survey method for this species, with 32 person-hours of effort resulting in one detection in the Mackay region (Ball 2004).
The presence of an active or inactive mud shelter (Section 3.5) or conclusive feeding sign (Section 3.6) are effective ways to identify a likely water mouse occurrence. The use of detection dogs also holds much potential. Follow-up trap or camera surveys are recommended in locations where water mouse mud shelters, feeding sign and/or positive scent indications from a detection dog are suspected (e.g. Ball 2004; Ball & Mitchell 2018).
The water mouse is not always detectable at known sites, suggesting unknown factors drive temporal variation in occurrence (Ball 2004; Gynther 2011).
Cameras monitoring water mouse shelters along the southern Queensland coast detected what appears to be water mouse torpor, with a frenetic feeding period just prior to the cold season followed by significantly reduced activity (Kaluza 2021 pers. comm.). Surveys for feeding sign, remote camera surveys, and box trap surveys may not detect this species during cooler months in this region.
The water mouse may also be temporarily absent from potential or confirmed habitat due to interannual, annual, monthly or daily wetland saturation conditions and prey availability (Woinarski et al. 2000; Gynther 2011), or recent disturbance from cyclone, extreme flooding and/or mangrove dieback (see Sections 4.2 and 4.3).
There are very few areas where water mouse presence and/or abundance trends are monitored, and none where they are systematically monitored using standardised methods and/or in relation to threat dynamics or the effectiveness of management actions. This creates challenges in understanding natural water mouse population dynamics, the severity of known and potential threats, and the effectiveness of threat mitigation programs.
The primary threat to the national water mouse population is from unsustainable urban and coastal development that removes, degrades, and fragments habitat and areas supporting recovery (Woinarski & Burbidge 2016; CITES 2019). Coastal development includes – but is not limited to – urban and industrial estates, mining, sea- and air-ports, and infrastructure to support the built environment. Land clearance, including for houses, buildings, roads and mines, is a recognised Key Threatening Process for threatened species in Australia.
Most proposed development activities in coastal Queensland and the Northern Territory – including urban, commercial, infrastructure, and agricultural development – must consider the water mouse as part of an impact assessment and approvals process. The extent to which the water mouse is, and may continue to be, affected by coastal development was highlighted in 2015 by the development of species-specific guidelines to assist with preparing referrals and impact assessments that may affect the water mouse.
Canal housing estates and other significant changes to the banks and flow of coastal waterways for coastal development are of particular concern for the water mouse (Van Dyck et al. 2006; Benfer et al. 2011; Kaluza 2013; 2016a; 2016e).
There is some indication that the water mouse has the capacity to persist in habitats where adjacent areas are converted for agriculture (Kaluza et al. 2016), but not where there has been rapid and extensive change to adjacent areas for urban and commercial development (Ball 2004; Van Dyck et al. 2006).
Compounding threats
Coastal development creates additional threats to water mouse persistence that compound the impacts of direct habitat loss. These include:
- Significant fragmentation due to the loss of linear coastal habitat strips and intrusive development into mangrove habitat over the last 100-200 years. The loss of connectivity between the Coomera River and other water mouse locations resulted in recent restrictions to gene flow and reduced fitness (Benfer et al. 2011). Coastal development also creates barriers to recolonisation after natural or human disturbance. There is a real risk of water mouse locations becoming isolated and genetically compromised on the central and southern Queensland coast (Benfer et al. 2011). The degree of water mouse population fragmentation from coastal development is unknown.
- Increased potential for habitat and water quality degradation due to stagnation of water, disturbance of acid sulfate soils, chemical leaching and waterway pollution, waste-water treatment, and increased use of insecticides for mosquito control (Ball 2004; Van Dyck et al. 2006; Gynther & Janetzki 2008; Gynther 2011; Duke et al. 2015; Kaluza 2019).
- Food resource depletion due to stormwater runoff changing water quality and flow (Ball et al. 2004; 2013; Kaluza & Bolzenius 2015), and to narrowing of the intertidal mangrove and saltmarsh zone (Kaluza 2013). In the Mackay region, an increase in common point stormwater discharge from urban development resulted in a significant decline in sesarmid crab abundance (Ball 2013), which appears to be the primary water mouse prey item in the region (Ball 2004).
- Loss and damage to critical shelters and habitat from increased levels of wave wash from vessels, and damage to free-standing mound shelters, saltmarsh cover and mangrove habitat by motor vehicles including recreational dirt bikes, four-wheel drives, and quad bikes (Burnham 2000; Kaluza 2013; 2016d; 2019; Pioneer Catchment Landcare 2020; Kaluza 2021 pers. comm.). Regular disturbance from other recreational activities may also increase the risk of structural damage to shelters and habitats through trampling (Burnham 2000; Kaluza 2019).
- Potential loss and degradation of freshwater wetland habitat due to excessive groundwater extraction or disturbance of natural hydrological regimes (Gynther 2011; Van Dyck & Gynther 2012).
- Alteration of natural tidal regimes due to the construction of bund walls and runnels, installation of tidal flow gates to convert intertidal areas to dry land for coastal development (Gynther 2022 pers. comm.), and dredging. Deepening of the Mourilyan Harbour channel in 1993 resulted in the abandonment of water mouse mounds at Inarlinga due to a significant change in the tidal lens (WMRG 2022).
- Artificial light may reduce nocturnal foraging activity, increase anxiety, and increase predation risk for this nocturnal rodent (Beier 2006; Bedrosian et al. 2013; Zhang et al. 2020).
- Potential increase in predation pressure from stray and/or domestic cats and European red fox in areas adjacent to urban developments (Kaluza 2021 pers. comm.; see Stobo-Wilson et al. 2022).
- Potential increase in competition for shelter sites by the larger black rat (Rattus rattus) in mangroves adjacent to urban developments (Stokes et al. 2009; Banks & Hughes 2012; Banks & Smith 2015).
Central and southern Queensland coast
The impacts of coastal development on the water mouse are most acute and ongoing in southeast Queensland and in the Mackay region where historic and current human population growth exerts significant pressure to expand and intensify the urban footprint (CITES 2019; ABS 2021). The Gladstone region is also an area of concern due to the concentration and anticipated expansion of the seaport and industrial infrastructure and activities (TMR 2018).
The water mouse has declined at Coomera Waters on the Gold Coast (Van Dyck et al. 2006) and there are no water mouse records from potential habitat within the highly urbanised Brisbane City local government area (LGA), despite adjacent and nearby LGAs – Moreton Bay, Sunshine Coast, Redland City, Gold Coast – supporting populations.
Occupied water mouse habitat along the Mackay coast occurs adjacent to areas that are subject to clearing and modification for housing and aquaculture (Ball 2004), but the water mouse tends not to survive long-term in areas where there is development inland from mangrove habitat (CITES 2019). This does not bode well for the future of the water mouse in the greater Mackay area, particularly with the additional threat of habitat loss due to coastal squeeze as sea levels rise (see Section 4.2).
It is very likely that historical coastal development has caused a significant unmonitored decline in the national water mouse population and undetected localised extirpations along the southern and central Queensland coast due to insufficient knowledge about this species’ occurrence and its response to coastal development. This situation was undoubtably exacerbated by the unrecognised management responsibilities of the land owners, managers and Custodians of areas with water mouse habitat.
Northern Australia
The impacts of coastal development on the water mouse along the north Queensland coast (i.e. Mackay to Cooktown) and in the Torres Strait are unknown but may be significant in the Townsville and Cairns regions.
Apart from a few localised areas (e.g. Darwin, Weipa), there is very little coastal development in Cape York, the Gulf of Carpentaria, and across the Top End of the Northern Territory (including the Tiwi Islands) and poor knowledge about the water mouse distribution.
The water mouse is at high risk of significant and ongoing declines due to rapid inundation of mangroves and other coastal habitats as sea levels rise with the progression of climate change (Russell & Hale 2009; Traill et al. 2011). Loss of wetland habitat due to sea-level rise caused by anthropogenic emissions of greenhouse gases is a recognised Key Threatening Process for threatened species in Australia.
Coastal environments, particularly mangroves, are dynamic and generally well adapted to changing sea-level conditions (Duke et al. 2015; Woodroffe et al. 2016; Woodroffe 2018; Saintilan et al. 2020) and the water mouse is able to adjust to gradual small-scale sea-level changes in southeast Queensland (Van Dyck & Gynther 2003). However, it is unlikely that mangrove communities – which are the primary feeding habitat for the water mouse – will be able to adapt to the rapid sea-level rises that are predicted over the next century, particularly along the southern Queensland coast with its low tidal range and restrictions on downstream movement of sediment due to dams (Lovelock et al. 2015; Saintilan et al. 2020).
Extensive areas of built environment along the east coast of Queensland will further restrict the potential for landward migration of mangroves and other coastal habitats as sea levels rise, leading to a significant reduction in the amount and connectivity of intertidal water mouse habitat (Traill et al. 2011). Inland migration of mangrove communities as sea levels rise may also be restricted or prevented in remote areas with severe impacts from pigs, smothering weeds and/or frequent fire in, and adjacent to, the upper tidal (Duke 2022 pers. comm.).
The rapid salination of extensive low-elevation coastal freshwater floodplains as sea levels rise is predicted to reduce the overall availability of water mouse habitat across northern Australia (Woinarski et al. 2003; Woinarski & Winderlich 2014; see Fig. 3d in Bayliss et al. 2018). Saltwater intrusion into floodplains of the Northern Territory may be exacerbated by water buffalo (Bubalus bubalis) eroding travel pads that pre-emptively connect large freshwater systems to tidal flows (ASRAC 2017).
Climate-induced mangrove dieback has the potential to impact vast stretches of water mouse habitat across northern Australia. Broadscale mangrove dieback occurred in the Gulf of Carpentaria in 1982 and again in 2015 (Duke et al. 2017; 2022). The dieback affected the upper mangrove zone, which is the primary habitat for water mouse elsewhere in Australia. Up to 74,000 ha of potential water mouse habitat across 1000 km of coastline was simultaneously lost during the 2015 event. The capacity for these mangrove ecosystems to recover depends on the occurrence of additional perturbations (cyclones, storms, floods, further droughts) over the following ten years (Duke et al. 2017).
The most likely cause of shoreline mangrove dieback in the Gulf of Carpentaria was a temporary but extended sea level drop of approximately 0.4 m for six months causing moisture stress in the upper mangrove zone during an unusually long period of dry El Niño conditions (Duke et al. 2017; 2022). Ocean and air temperatures and evaporation rates are projected to increase over the next few decades (Moise et al. 2015), increasing the likelihood of future severe and extended hot and dry conditions across parts of Northern Australia (Dai 2013). Broadscale shoreline mangrove dieback is a previously unrecognised vulnerability of mangrove communities (Duke et al. 2017; 2022) and the water mouse to climate change. Dieback of seaward fringing mangroves from ‘drowning’ during La Niña conditions (Duke et al. 2022) may also impact the water mouse through increased exposure to wave and wind action.
Mangrove dieback in the upper tidal zone, potentially due to pig activity, has been recorded from Muralag/Prince of Wales Island in the Torres Strait (Fell et al. 2019), and mangrove dieback due to Phytophthora spp. infection has been recorded in the Normanby River area of Princess Charlotte Bay in far north Queensland, in the Northern Territory, and possibly also in the Gladstone area of the central Queensland coast (Weste et al. 1982).
The upper mangrove zone where the water mouse occurs is generally protected from intense wave action caused by cyclones and storms, and the water mouse appears to be well adapted to infrequent and temporary inundation from flood and storm surge in southeast Queensland (Van Dyck 1994; Kaluza 2013; Kaluza 2021 pers. comm.). However, the tropical coast from Mackay to Broome – excluding the islands of the Torres Strait – is subject to intense cyclones (Bureau of Meteorology Cyclone Tracks) that can cause damaging storm surges well above the highest astronomical tide (e.g. Yasi: Queensland Government 2012) and extreme wet season floods that can inundate vast areas of the lower catchments for long periods of time (Warfe et al. 2011). Damaging cyclones and east coast lows, and extreme seasonal floods, are anticipated to increase in frequency as climate change progresses (Knutson et al. 2010, 2020; Parker et al. 2018; Tabari 2020).
Prolonged, frequent and/or extreme inundation of water mouse habitat in is likely to result in high rates of mortality with localised declines and extirpations. The resilience of the water mouse to prolonged and extreme inundation events, and its capacity to disperse and recolonise impacted areas, is unknown.
Figure 20: A water mouse on top of its mud shelter during a flood caused by an east coast low and high tide.

Source: © Janina Kaluza (Great Sandy Strait in 2015).
European red fox: central and southern Queensland coast
The introduced European red fox (Vulpes vulpes) – a rodent predator (Stobo-Wilson et al. 2021) – is a primary threat to the water mouse in areas where the distributions of these two species overlap i.e. along the central and southern Queensland coast including on K’gari/Fraser Island, Minjerribah/North Stradbroke Island and Bribie Island. Foxes are an emerging threat on K’gari/Fraser Island and South Stradbroke Island, where they established as recently as 2012 and 2013 respectively (Allen et al. 2017). Fox density is highest in areas with dense human populations (Stobo-Wilson et al. 2022).
European red fox sign or presence on cameras has been reported during targeted water mouse surveys on the Gold Coast and Sunshine Coast, in the Great Sandy Strait, and on the central Queensland coast (Burnham 2000; Kaluza et al. 2016; White & Power 2016; Sutherland 2017; Kaluza 2018; Pioneer Catchment Landcare 2020). European red foxes dismantled 88 % (42/48) of monitored active water mouse shelters in the Maroochy River Conservation Park and adjacent areas following rapid urban development nearby and a thorough ‘wild dog’ control program in the surrounding region (Kaluza et al. 2016; Kaluza 2021 pers. comm.). The European red fox is also suspected to have damaged water mouse shelters at Wangoolba Creek on K’gari/Fraser Island (Kaluza 2016g; Allen et al. 2017). The loss of active water mouse shelters from saltmarsh on the Gold Coast is believed to be due to European red fox and/or cat predation (Boody 2021 pers. comm.).
Predation by European red fox is a recognised Key Threatening Process for threatened species in Australia.
Figure 21: Camera trap detections of European red foxes disturbing and dismantling water mouse mounds along the southern Queensland coast.



Source: © Janina Kaluza (top @ Great Sandy Strait in 2016 & 2015; bottom @ Maroochy River in 2016).
Feral pig: all areas
Predation and dismantling of critical shelters by the introduced feral pig (Sus scrofa) are major concerns for the water mouse across its distribution. There is a high degree of overlap in distribution and areas occupied by the two species, and pigs are able to detect and dig into compacted mud to prey on small vertebrates (Redhead & McKean 1975; Van Dyck & Gynther 2003; Gynther 2011; Kaluza 2018; Pioneer Catchment Landcare 2020). The feral pig continues to expand its distribution and increase its abundance across northern Australia including along the north and east Queensland coasts (Bengsen et al. 2017).
Feral pigs have been recorded dismantling critical shelters and preying on water mouse at multiple locations along the east Queensland coast (Van Dyck & Gynther 2003; Kaluza 2021 pers. comm.) and evidence of feral pig damage to water mouse habitat has also been observed in these regions (Gynther 2011; White & Power 2016; Kaluza 2018; Pioneer Catchment Landcare 2020). Feral pig rooting causes significant structural damage to water mouse shelters and habitat, and to natural hydrological flows (Redhead & McKean 1975; Burnham 2000).
Predation and habitat loss by feral pigs is a recognised Key Threatening Process for threatened species in Australia.
Figure 22: Pig damage to water mouse habitat (left) and a mud mound shelter (right).


Source: © Ian Gynther (Pumicestone Passage).
Cat: all areas
Predation by cats (Felis catus) may have a negative impact on the water mouse population in some locations and there is some concern that control programs for European red fox may result in increased predation pressure from cats where these two predators co-occur in water mouse habitat (Boody 2021 pers. comm.). The water mouse falls within the preferred prey size range of feral cats (Woolley et al. 2019), although feral cat predation is believed to be a minor concern in the remote Northern Territory due to a limited overlap in the ecological communities occupied by the two species (Woinarski et al. 2006; Woinarski & Burbidge 2016).
Predation by feral cats is a recognised Key Threatening Process for threatened species in Australia.
The loss and degradation of water mouse habitat in floodplains and coastal swamps is likely to have occurred in the past for the development of crop fields, e.g. sugar cane (Ovington 1978; Kaluza et al. 2016) and there are plans for Aquaculture Development Areas immediately adjacent to intertidal areas along the north and central Queensland coasts, which may restrict inland migration of water mouse habitat as sea levels rise. Aquaculture developments across the water mouse distribution have the potential to impact this species through habitat loss and altered hydrology unless adequately regulated to avoid such impacts. Land clearance, including for crops, is a recognised Key Threatening Process for threatened species in Australia.
Industrial waste, sewage, and thermal effluent from industrial and urban developments in coastal areas are a recognised threat to the water mouse and its habitat (Van Dyck & Gynther 2012).
Chemical
The impacts of regular mosquito control (spray) programs in mangrove, saltmarsh and wetland habitats near urban areas may impact the water mouse through bioaccumulation within their crab and invertebrate prey (Van Dyck et al. 2006) and through physical damage to habitat.
Runoff from Diuron and other residual herbicides that are used during crop production can cause dieback of grey mangrove (Shearer 2004), which is a significant habitat component for the water mouse. Historical widespread use of residual herbicides may have led to the loss and degradation of water mouse habitat along the east coast of Queensland. The use of residual herbicide is now controlled, although contraindicative use may continue and have adverse impacts in some areas.
Oil spill
As intertidal species, the water mouse and its invertebrate prey may occasionally be impacted by widespread oil spills e.g. the 2009 oil spill off the coast of Minjerribah/North Stradbroke Island (Burnham 2000; Gynther 2011). The risk of oil spill impact is highest from the Torres Strait to Coomera due to the presence of the Great Barrier Reef and/or a high density of shipping (Australian Maritime Safety Authority).
Port Melville on the Tiwi Islands may represent a significant risk to water mouse on the Tiwi Islands due to its extensive capacity as a diesel storage supply base and lack of environmental regulation. The response of water mouse populations to coastal oil spills is unknown.
Plastic
Coastal pollution from drifting plastics and other waste is a significant concern for coastal areas in northern and eastern Australia (Reisser et al. 2013; Galaiduk et al. 2020). The long-term impact of coastal plastic pollution on the water mouse, and its habitat and prey are unknown.
The water mouse requires intact shelters and a cover of vegetation to avoid predation. In southeast Queensland, free-standing shelter mounds decline in overgrazed areas (Gynther 2011; Kaluza 2013, 2018). Feral cattle (Bos taurus), water buffalo, and horses (Equus caballus) all have the potential to damage and degrade large areas of water mouse habitat through overgrazing and trampling, particularly in remote areas (Ovington 1978; Burnham 2000; Woinarski 2006; Gynther 2011; ASRAC 2017). Habitat degradation by feral cattle, buffalo and horses is a recognised Key Threatening Process for threatened species in Australia under novel biota and their impact on biodiversity.
Figure 23: Significant cattle pugging and mud disturbance in water mouse habitat.


Sources: © Janina Kaluza (left in 2012) and © Ian Gynther (right @ Coomera River).
Fire is known to destroy and degrade water mouse shelters within saltmarsh communities (Van Dyck & Gynther 2003) and fire removes supratidal and intertidal vegetation cover, exposing the water mouse to increased predation pressure (Burnham 2000; Kaluza 2019). Extensive and frequent fire intrusion into saltmarsh and other supratidal habitats is likely to increase in extent and severity across the known distribution of the water mouse as climate change progresses (Ward et al. 2020; State of Queensland 2021). Fire is unlikely to impact mangrove habitat (Woinarski & Winderlich 2014).
Figure 24: Burnt water mouse mud mound and surrounding habitat.

Source: © Ian Gynther (Donnybrook in the Pumicestone Passage).
Weeds have the potential to degrade water mouse habitat, particularly mimosa (Mimosa pigra) and olive hymenachne (Hymenachne amplexicaulis) in the Northern Territory, and lantana (Lantana camara) and groundsel bush (Baccharis halimifolia) in southeast Queensland (Woinarski et al. 2003; Kaluza 2016c; ASRAC 2017). Introduced pasture grasses in northern Australia fuel intense and unmanageable seasonal fires (Setterfield et al. 2010) that can spread into water mouse habitat. Invasion of northern Australia by Gamba Grass and other introduced grasses, including olive hymenachne, is a recognised Key Threatening Process for threatened species in Australia.
The presence and relative impact of threats to the water mouse vary significantly across this species’ broad distribution. Each of the threats has been assessed to determine the risk posed to the regional water mouse population using a risk matrix (Table 2). The risk matrix considers the likelihood of an incident occurring and the consequences of that incident. Table 3 provides an indicative summary of relative threat risk for each of five regions across the water mouse distribution.
Table 2: Risk matrix
Likelihood of occurrence | Consequences |
Not significant | Minor | Moderate | Major | Catastrophic |
Almost certain | Low | Moderate | Very High | Very High | Very High |
Likely | Low | Moderate | High | Very High | Very High |
Possible | Low | Moderate | High | Very High | Very High |
Unlikely | Low | Low | Moderate | High | Very High |
Rare or Unknown | Low | Low | Moderate | High | Very High |
Table 3: Indicative summary of threat impacts across the water mouse distribution.
Region | Risk level | Threats |
South-east Qld: Coomera to Noosa | Very high | Coastal development, rapid sea-level rise, European red fox, chemical pollution. |
High | Feral pig, cat, oil spill, cropping. |
Moderate | Prolonged and/or extreme inundation, cattle, fire, aquaculture. |
Low | Mangrove dieback, weeds, horse. |
Unknown | Plastic pollution. |
Not relevant | Saltwater intrusion, water buffalo. |
South-central Qld: Noosa to Gladstone | Very high | European red fox, feral pig. |
High | Coastal development, rapid sea-level rise, chemical pollution, cat, cattle, oil spill. |
Moderate | Aquaculture, prolonged and/or extreme inundation, fire. |
Low | Cropping, weeds, horse. |
Unknown | Plastic pollution, saltwater intrusion, mangrove dieback. |
Not relevant | Water buffalo. |
Central Qld: Gladstone to Cannonvale, including Mackay | Very high | Coastal development, rapid sea-level rise, feral pig, chemical pollution. |
High | Prolonged and/or extreme inundation, cropping, European red fox, cat, oil spill. |
Moderate | Cattle, aquaculture. |
Low | Fire. |
Unknown | Horse, plastic pollution, weeds, mangrove dieback. |
Not relevant | Saltwater intrusion, water buffalo. |
North Qld: Cannonvale to Cooktown | Very high | Feral pig. |
High | Coastal development, rapid sea-level rise, cat, prolonged and/or extreme inundation, cropping, chemical pollution. |
Moderate | Oil spill, aquaculture. |
Low | Fire, cattle, horse. |
Unknown | Plastic pollution, weeds, saltwater intrusion. |
Not relevant | European red fox, mangrove dieback, water buffalo. |
Remote northern Australia: Cooktown (Qld) to Broome (WA), including the Tiwi Islands | Very high | Feral pig, mangrove dieback. |
High | Prolonged and/or extreme inundation, saltwater intrusion. |
Moderate | Fire, cattle, water buffalo, rapid sea-level rise, weeds. |
Low | Coastal development, oil spill, chemical pollution, aquaculture, cat, horse. |
Unknown | Plastic pollution. |
Not relevant | European red fox, cropping. |
Regions with the highest risk of water mouse extirpation all occur in Queensland: from Coomera to the Noosa River, the Fraser Coast, and along the Mackay coast. In these areas, coastal development to support significant human population growth (ABS 2021) is expanding and intruding into – and/or fragmenting – water mouse habitat; or creating landward barriers that will result in significant habitat coastal squeeze in the future as sea levels rise.
Areas of considerable concern include:
- Along the Pimpama River and elsewhere within the greater Gold Coast City Council LGA due to very high rates of human population growth and associated ongoing development pressure.
- Islands of southern Moreton Bay due to unsustainable coastal development and associated pressures including adverse recreational activities and feral predators. Projected sea level rise and increasing mud deposition are also a concern in this area.
- Minjerribah/North Stradbroke Island due to coastal developments associated with increased tourism.
- The Pumicestone Passage and Bribie Island due to a recent and significant expansion of the Caloundra urban footprint in the north and an increase in tourism and associated development on Bribie Island to the south.
- The Maroochy River due to urban expansion at Twin Waters, Mudjimba and Bli Bli.
- The Gladstone-Curtis Island port area due to anticipated expansions.
- All water mouse locations along the east Queensland coast that are seaward of development barriers that will impede coastal water mouse habitat migration inland as sea levels rise (see Section 5.2.2, Action 2.3).
Additional areas along the east coast of Queensland may be considered under pressure in the future as the water mouse is detected in new locations (e.g. the Cairns region, Townsville) and/or unregulated and unsustainable development occurs to accommodate population growth.
As a threatened species, the water mouse is managed by Commonwealth and State or Territory Governments across its range to mitigate the impacts of coastal development through appropriate conditioning of approvals and ongoing regulation of development conditions.
Approximately 30 % of the Maroochy River floodplain is being restored from former cane fields to tidal wetlands (i.e. water mouse habitat) under the Blue Heart Sunshine Coast program. This program has the potential to restore and create new climate-resilient water mouse habitat as well as consolidate and conserve existing habitat at an important water mouse location that is under pressure from development (Webb 2021 pers. comm.).
Locations where threats to water mouse are explicitly acknowledged and actively managed include:
- Gold Coast City Council reserves (McCoys Creek, Pimpama, Coomera) where threats to the water mouse (e.g. coastal development, European red fox, fire) have been investigated and are actively managed to reduce impacts as outlined in a plan of management (City of Gold Coast 2021; Adkins 2021 pers. comm.). The water mouse is one of five fauna species ranked by the Gold Coast City Council as the highest priority for management intervention (Adkins 2021 pers. comm.).
- The Maroochy River in southeast Queensland, where European red fox monitoring and control is implemented by the Sunshine Coast Council in conjunction with the Kabi Kabi Traditional Owners to reduce predation pressure on the water mouse. The water mouse population is monitored along the Maroochy River to assess the response to fox management (SCC 2020).
- Bustard Bay on the central Queensland coast, where private graziers have installed exclusion fencing to remove cattle long-term from water mouse habitat (Burnett Mary Regional Group NRM 2019).
- Cape Palmerston National Park, Sandringham Bay Conservation Park, and Skull Knob Conservation Park on the central Queensland coast, where a European red fox management program was implemented at locations with recent water mouse detections (Pioneer Catchment Landcare 2020).
- Gurruwiling/Arafura Swamp in Arnhem Land in the Northern Territory, where although the water mouse has not been detected since the year 2000, potential threats from feral herbivores and fires are actively managed long-term by the Arafura Swamp Rangers Aboriginal Corporation, and targeteed surveys are implemented to detect the water mouse (ASRAC 2017, 2018).
Additional locations where an active management plan is in place that acknowledges the known or potential occurrence of water mouse and indirectly considers impacts to this species in a larger framework of broader landscape-level management include:
- Naree Budjong Djara on Minjerribah/North Stradbroke Island in south-east Queensland (QYAC QPWS 2020).
- Sarina Beach near Mackay on the central Queensland coast (MRC 2022).
- Kakadu National Park in the Northern Territory (Woinarski & Winderlich 2014; Director of National Parks 2016).
The water mouse is earmarked for consideration during review of the Pumicestone Passage Action Plan and programs (Webb 2021 pers. comm.).
In 2021, broad management plans were under development for K’gari/Fraser Island and for the Great Sandy Strait Ramsar wetland area.
It is challenging to attribute a definitive tenure to most water mouse locations due to their occurrence in intertidal areas, which are often the defined boundary between management and custodianship tenures. Tenure information and associated population information for water mouse locations is available in Appendix A.
With few notable exceptions, the areas where the water mouse is most abundant and regularly detected are on the boundary of, or at the conjunction of, marine and terrestrial protected areas, joint‑management Native Title, and/or Commonwealth Department of Defence areas (Table 4). These land tenures afford significant long-term protection from the primary threat to the water mouse, i.e. coastal development. Effective management and monitoring of water mouse in these locations is required to address water mouse shelter damage and excessive predation by introduced predators, trampling and overgrazing by large herbivores, and loss of habitat and critical shelters to fire. There may be future concerns about water mouse population viability due to coastal squeeze from sea-level rise in marine protected areas adjacent to the built environment, e.g. parts of The Great Barrier Reef Marine Park.
There is significant overlap between recorded water mouse locations and internationally recognised Key Biodiversity Areas (BirdLife International 2021):
- Moreton Bay and Pumicestone Passage, Cooloola and Fraser Coast, Great Sandy Strait, and Repulse Bay to Ince Bay in Queensland.
- Arafura Swamp, Alligator River Floodplains (historical record: 1903), Tiwi Islands, and Anson Bay, Daly and Reynolds River Floodplains in the Northern Territory.
Table 4: Occurrence of water mouse in protected and heritage areas.
Type | Name |
Marine park and associated fish habitat areas | Moreton Bay, Great Sandy, Great Barrier Reef, Great Barrier Reef Coast. |
National park | Naree Budjong Djara, Bribie Island, Pumicestone, Mooloolah River, Great Sandy, Poona, Eurimbula, Cape Palmerston, Cape Hillsborough, Kakadua. |
Indigenous protected area | Djelk. |
Conservation park | South Stradbroke Island, Myora, Bullock Creek, Maroochy River, Eudlo Creek, Maroochy Wetlands, Coolum Creek, O’Reagan Creek, Sandringham Bay, Bakers Creek, Skull Knob. |
Nature refuges and environment reserves | Edward Corbould Reserve and Retreat No. 2 Nature Refuge, Maroochy Wetland Sanctuary, Maroochy River Environment Reserve, Coolum Creek Nature Refuge, Coolum Creek Environment Reserve. |
Resources reserve | Eurimbula. |
World and national heritage areas | K’gari (Fraser Island), Great Barrier Reef, Wet Tropics of Queensland, Kakadu National Parka, Great Sandy (proposed). |
Commonwealth heritage area | Wide Bay Military Reserve. |
Ramsar wetland | Moreton Bay, Great Sandy Strait, Kakadu National Parka. |
Nationally important wetland | Moreton Bay, North Stradbroke Island, Bribie Island, Pumicestone Passage, Upper Pumicestone Coastal Plain, Coolum Creek and Lower Maroochy River, Noosa River Wetlands, Great Sandy Strait, Wide Bay Military Training Area, Fraser Island, Great Barrier Reef Marine Park, Bustard Bay, Colosseum Inlet-Rodds Bay, Port Curtis, Fitzroy River Delta, Sarina Inlet-Ince Bay, Sandringham Bay-Bakers Creek Aggregation, Proserpine-Goorganga Plains, Port of Cairns and Trinity Inlet, Arafura Swamp, Kakadu National Parka. |
a Historical record from 1903
The water mouse is afforded local community protections in the Barron River delta near Cairns due to this area being a focus for a multi-partner supported river restoration program (Barron Catchment Care 2017).
The long-term vision for the water mouse is that its distribution, population trends and threats are understood, and threats are effectively addressed to ensure the ongoing decline in the national population is stabilised and shows recovery despite anticipated future impacts of climate change.
The aim of the water mouse recovery plan is to implement actions that will reduce the impact of the primary known threat to water mouse (i.e. coastal development) and to increase knowledge about the species and its threats to ensure effective recovery actions are implemented. Specifically, the three objectives of the plan are:
- Significant impacts on the water mouse from coastal development and sea-level rise are effectively mitigated through sustainable development and habitat restoration initiatives.
- Current and potential future threats to the water mouse are better understood and mitigated through research and adaptive management.
- The distribution and ecology of the water mouse is clarified, with effective management and monitoring actions implemented where it occurs. This includes areas primarily focused on conservation as well as locations with alternative primary objectives.
The following strategies are designed to meet these objectives within the 10-year lifespan of this recovery plan:
- Strategy 1: Ensure activities and developments in coastal areas within the current and future modelled water mouse distribution are adequately assessed, regulated, and managed to ensure no detrimental short-, medium-, or long-term impacts on the national population.
- Strategy 2: Map water mouse habitat and locations at a fine scale to ensure relevant land managers and Custodians are identified and engaged in water mouse recovery.
- Strategy 3: Develop clear and adaptive communications and implement tailored engagement processes to ensure relevant land managers and Custodians are effectively engaged in water mouse detection, management and monitoring.
- Strategy 4: Implement targeted water mouse detection surveys in areas of potential habitat across the water mouse distribution.
- At confirmed water mouse locations:
Strategy 5: Support current and future land managers and Custodians to include the water mouse in adaptive land management plans that support persistence and recovery by identifying local threats to this species, and implementing actions to address them.
Strategy 6: Ensure effective water mouse population monitoring occurs to enable local and national population trends, impacts of threats, and effectiveness of management actions to be assessed.
Strategy 7: Investigate water mouse ecology and detectability, and the impact of threats to the national population.
The water mouse occurs, or may occur, on Country of many Aboriginal and Torres Strait Island Peoples (Table 1). Interests and opportunities for Indigenous Peoples must be incorporated into actions outlined in this recovery plan (Thompson et al. 2020), including consultation and engagement protocols that are relevant to each organisation. There are significant opportunities for Indigenous Peoples to lead and co-lead recovery actions outlined in this plan.
5.2.1 Strategy 1: Adequate regulation and management of coastal development
All developments with the potential to have a negative impact (i.e. a significant impact) on a known or undetected water mouse population must be appropriately assessed and regulated to ensure water mouse locations under pressure remain resilient, recovery of the national population is not impeded, and a sustainable coastal development approach is implemented.
Table 5: Actions to ensure activities and developments in coastal areas within the modelled distribution of the water mouse are adequately assessed and regulated (Strategy 1).
Action No. | Action Description | Action Details | Performance criteria |
1.1 | Development proposals and actions within the modelled water mouse distribution and adjacent areas supporting recovery are appropriately assessed and regulated. Responsibility: • Local, State & Commonwealth planning departments •Local, State & Commonwealth, environment departments •Ecological experts within the Water Mouse Recovery Team | Commonwealth and State planning departments, and Local Councils to include current (Map 1) and future (Action 2.3) modelled distributions of the water mouse in all local and regional planning processes. All development proposals and activities within the current and future modelled water mouse distribution that may result in a decline in the national population (Sections 4.1, 4.6 and 4.7) – alone or in conjunction with other activities – must be referred to the Commonwealth for assessment and the assessment must be reviewed by a member of the Water Mouse Recovery Team with expertise in water mouse ecology and threats. Adequate assessment and approval condition-setting of coastal developments requires an intricate understanding of water mouse ecology and threats. In some locations this information is sparse and a precautionary approach is required until further surveys and targeted research programs are completed. The Water Mouse Recovery Team is best placed to analyse potential development impacts using the most up-to-date water mouse ecology and threat information. Referral guidelines were developed for the water mouse in 2015 to support developers and assessors. While still relevant to supporting referral development, all referred actions within the modelled water mouse distribution must now be assessed against the information and actions outlined in this recovery plan. The water mouse recovery plan supersedes the referral guidelines. Under section 139 of the EPBC Act, the Minister must not act inconsistently with a recovery plan when deciding whether or not to approve the taking of an action. The cumulative impacts of coastal development (see Section 4.1) must be addressed when considering potential impacts on the water mouse, and sufficient consideration must be given to the following knowledge gaps about the water mouse: • Occurrence outside southeast Qld (Section 3.3). • Habitat attributes outside southeast Qld (Section 3.4). • Detectability, intermittent occupancy and areas supporting recovery (Sections 3.4, 3.11, 3.12, 4.3 and 4.4). • Dispersal and capacity to maintain gene flow (Section 3.8). • Susceptibility or resilience to increased cyclone and storm activity (Section 4.4). • The predicted migration, viability, and connectivity of coastal habitat as sea-levels rise (Section 4.2). • The capacity for inland and degraded areas to become habitat in the future (Sections 3.4 and 4.2). Where there is uncertainty, a precautionary approach must be implemented until knowledge gaps can be addressed. | Proportion of proposed and approved coastal developments within the modelled water mouse distribution that are reviewed by a member of the Water Mouse Recovery Team. Proportion of proposed and approved coastal developments within the modelled water mouse distribution that appropriately consider each of the knowledge gaps outlined and condition the development accordingly using a precautionary approach. |
1.2 | Adequately account for detrimental impacts of offsets when considering how to mitigate coastal development impacts. Responsibility: •Developers •Local, State & Commonwealth environment departments. | Offsets are unlikely to be a viable option to address impacts to the national water mouse population in most circumstances due to the linear coastal distribution of this species, its sensitivity to fragmentation and genetic isolation along the urbanised east Queensland coast, and its occurrence in dynamic coastal areas. Offsets may only be considered an option to compensate for development impacts to the water mouse under the following conditions: • The anticipated impact on the national water mouse population from the development can be accurately estimated, including consideration for fragmentation and future habitat migration under sea-level rise, and • It can be demonstrated that the offset location has a water mouse population that is below capacity due to degradation and/or one or more threats that are unlikely to be alleviated without intervention, and the degradation or threat/s can be removed or managed indefinitely by the proponent or a partner organisation, and • It can be demonstrated that water mouse habitat at the offset location has a high capacity to successfully migrate inland within the offset boundary as sea-levels rise with climate change, and • There are no foreseeable threats to water mouse and its habitat at the offset site that will not be managed or mitigated, and • Long-term protection of the offset site as a water mouse refuge is legally secured, and • Prior to the development occurring, management of the offset location results in a demonstrable sustained increase in water mouse abundance that is greater than the estimated decline in abundance at the development site. Offsets based on habitat area will not be considered appropriate for reducing impacts on the water mouse until a greater understanding about water mouse habitat requirements, densities, and responses to threats is achieved through implementing the research, monitoring and management actions outlined in this plan. The Water Mouse Recovery Team, in conjunction with the Commonwealth Environmental Approvals Department, will be best placed to determine when this offset strategy may become applicable. The feasibility of translocating impacted individuals from a development site to an approved offset site should be considered in conjunction with species and translocation experts, trialled where a conservation gain is anticipated, and comprehensively monitored to assess the long-term (i.e. > 10 year) impact. | Proportion of coastal developments with offsets for the water mouse that were able to show a demonstrable and sustained increase in water mouse abundance that was equivalent to the estimated decline in water mouse abundance at the development site, prior to the development occurring. |
1.3 | Appropriate regulation of coastal developments through compliance Responsibility: Local, State & Commonwealth environment departments | All regulated coastal developments are monitored to ensure each proponent complies with the conditions of the development approval that relate to avoiding water mouse impacts. All breaches of development approval conditions that may impact the water mouse are to be penalised in accordance with the costs of remediating the impact of the breach. Detrimental impacts from such breaches must be urgently remediated by the development approval holder or their delegate via consultation with water mouse experts. | Proportion of coastal developments where all conditions relating to water mouse impact mitigation are confirmed by regulators to be compliant. |
1.4 | Ensure a sustainable development approach to coastal development Responsibility: Local, State and Commonwealth planning and environment departments | An ecologically sustainable development approach for coastal areas is required to ensure the long-term viability of the water mouse. Commonwealth and State planning departments, and Local Councils are to include current (Map 1) and future (Action 2.3) modelled distributions of the water mouse in all local and regional planning processes. Spatial adjustments to proposed developments directly adjacent to and within the modelled water mouse distribution are likely to be required to effectively mitigate potential impacts to the national population. Regional approaches to sustainable development (e.g. Strategic Assessments) under a climate change scenario are encouraged to provide developers with clarity about restrictions on coastal development opportunities in areas where the water mouse is known or likely to occur. All coastal development actions directly adjacent to and within the modelled water mouse distribution must be assessed within the context of all past, current and future actions and activities within the region that may impact the water mouse, to effectively mitigate the risk of ongoing water mouse declines caused by cumulative actions. | Proportion of the water mouse distribution on the east coast of Queensland that is managed under a regional development plan that reduces or removes long-term impacts to the water mouse from coastal development. |
Implementing the actions outlined in Table 5 will ensure Australia has the capacity to meet national and international obligations for biodiversity conservation and sustainable development along the east and northern coasts, including within wetlands of international importance.
Fine-scale mapping and regular review of water mouse distribution and habitat is required to provide regional planners and developers with representative and up-to-date information about water mouse distribution and potential occurrence. The initial mapping program will also enable water mouse land managers and Custodians to be identified and engaged in recovery actions, reducing the risk of loss due to unidentified risk and land management responsibility.
Table 6: Actions to map water mouse habitat and locations at a fine scale (Strategy 2).
Action No. | Action Description | Action Details | Performance criteria |
2.1 | Map water mouse distribution at a fine scale. Responsibility: Water Mouse Recovery Team. | Using the Regional Ecosystem (QLD) and NVIS (national) mapping frameworks as a basis, develop a representative fine-scale spatial map layer of areas with known, likely and potential water mouse habitat and areas supporting recovery. Additional considerations for developing this layer include highest astronomical tide and/or mean high water springs tidal modelling, high resolution digital elevation models and LiDAR-derived elevation and structure information, and high to very high resolution multi-spectral satellite imagery to assist with identifying intertidal and damp freshwater areas. Incorporate the fine-scale distribution layer into the Commonwealth Protected Matters Search Tool and other regulatory databases. Update the fine-scale water mouse distribution layer every 3 years for the life of the recovery plan, to support adaptive management and planning as new ecological insights occur. | Actions completed (yes/no) |
2.2 | Identify current land managers and Custodians. Responsibility: Water Mouse Recovery Team. | Use the most recent water mouse distribution (including areas supporting recovery) map to identify all land managers and Custodians with areas of known, likely and/or potential water mouse habitat. Review updated maps every 3 years in relation to partner management areas to ensure all relevant parties are, or remain, engaged. | Actions completed (yes/no) |
2.3 | Map predicted future habitat locations and locations under pressure as sea levels rise. Responsibility: Research institutions. | Develop spatially explicit time-series probability maps of predicted water mouse habitat locations across its distribution in relation to coastal habitat inland migration as sea levels rise. Highlight areas where coastal squeeze (due to coastal development) is highly likely to result in the loss or decline of water mouse habitat and connectivity in the future. | Actions completed (yes/no) |
2.4 | Identify future land managers and Custodians. Responsibility: Water Mouse Recovery Team. | Compare current and future models of water mouse habitat locations to identify additional partners that may be relevant to water mouse recovery in the future. | Action completed (yes/no) |
5.2.3 Strategy 3: Develop communications processes and implement effective partner engagement
The initiation and ongoing funding of a registered national Water Mouse Recovery Team is fundamental to effective water mouse recovery. The primary role of the Water Mouse Recovery Team is to manage recovery efforts through effective information and communication dissemination among the many and diverse partners. The communications and data management actions and processes outlined in Table 7 are required to ensure information flows freely among partners.
Table 7: Actions to develop clear and adaptive communications and implement tailored engagement processes (Strategy 3).
Action No. | Action Description | Action Details | Performance criteria |
3.1 | Create and maintain a Water Mouse Recovery Team. Responsibility: Commonwealth Environment Department. | Develop and maintain a Water Mouse Recovery Team. The team is to include ongoing representation from Indigenous Peoples to ensure cultural interests and knowledge are appropriately considered and managed. | Action completed (yes/no) |
3.2 | Develop location-relevant water mouse information material. Responsibility: Water Mouse Recovery Team with the support of NRM bodies and Indigenous Land Councils. | Develop brief and engaging location-relevant water mouse information brochures and/or videos for distribution to landowners, managers, and Custodians for known and modelled water mouse habitat. The information format is to include imagery of the whole animal, its local habitat associations and attributes, relevant ecology, and information about local threats, active community groups, local actions, and potential funding sources. All communications are to use culturally appropriate language. | Action completed (yes/no) |
3.3 | Engage landowners, managers, and Custodians, and other relevant partners in water mouse recovery. Responsibility: •Water Mouse Recovery Team. •NRM bodies, Land Councils. •Conservation organisations. | Develop and facilitate relationships among landowners, managers and Custodians, and other partners relevant to water mouse recovery. Encourage landowners, managers, and Custodians to be involved in water mouse recovery actions including surveys, monitoring and threat management. Distribute relevant information among partners to promote engagement and information sharing. | Proportion of landowners, managers and Custodians engaged. Proportion of other partners engaged. |
3.4 | Raise community awareness Responsibility: •NRM bodies, Land Councils, Aboriginal Corporations and Indigenous Rangers, Landcare Groups •Conservation organisations. •Land managers and Custodians. •Water Mouse Recovery Team. | Raise awareness about the water mouse across communities using culturally appropriate content to increase engagement and the likelihood of incidental water mouse detections or signs being reported. Engage local community members including citizen scientists in water mouse recovery activities where appropriate. Investigate the potential for a captive water mouse population to increase connection with the public. Develop and communicate a process for reporting potential and confirmed incidental water mouse detections to the Water Mouse Recovery Team. | Proportion of water mouse locations where community engagement activities have occurred. |
3.5 | Compile shareable water mouse recovery information in a centralised location. Responsibility: Water Mouse Recovery Team. | Develop or co-opt a data management system to record all relevant water mouse recovery information in a single accessible location. Create or facilitate a water mouse identification support program to assist on-the-ground recovery partners. | Actions completed (yes/no) |
5.2.4 Strategy 4: Implement targeted water mouse detection surveys
There are significant gaps in knowledge about the distribution and occurrence of water mouse across its known and potential distribution, hindering the capacity to identify at-risk locations, prioritise recovery actions, and develop a clear understanding of overall extinction risk.
Table 8: Actions to support targeted water mouse detection surveys in areas of potential habitat (Strategy 4).
Action No. | Action Description | Action Details | Performance criteria |
4.1 | Develop and disseminate locally relevant technical information to support field survey programs. Responsibility: Water Mouse Recovery Team. | Compile clear and concise location-relevant technical information to support survey programs for the water mouse in a culturally appropriate format. Technical information is to include key water mouse identification features, local habitat associations and attributes, survey methods, genetic sample collection methods, and permit requirements. Distribute technical information to partners involved in water mouse survey programs to increase the likelihood of water mouse detection during field programs. Review and update technical information as new information comes to light. | Action completed (yes/no) |
4.2 | Develop a concise water mouse survey reporting mechanism. Responsibility: Water Mouse Recovery Team. | Develop a process and easy-to-use template for reporting targeted water mouse survey program methods and outcomes to the Water Mouse Recovery Team. | Action completed (yes/no) |
4.3 | Incorporate targeted water mouse detection survey methods into general fauna survey programs. Responsibility: •Land managers and Custodians. •Ecologists. | Appropriate survey methods to target water mouse are to be included in all general fauna survey programs that occur within the modelled distribution for this species between Broom’s Head in New South Wales and Roebuck Bay in Western Australia (Map 1), with consideration for access, human and animal safety, and access to suitable survey equipment. Additional animal ethics and/or survey permits may be required. Targeted surveys for water mouse are to include techniques outlined in the information brochures developed in Action 4.1, incorporating key information from Section 3.11, and with consideration for intermittent detectability (Section 3.12). | Proportion of general fauna survey programs within the modelled water mouse distribution include targeted water mouse methods. |
4.4 | Implement targeted water mouse detection surveys. Responsibility: •Land managers and Custodians. •Ecologists. | Survey programs specifically targeting water mouse are to be implemented as a priority, where feasible and safe (see below), at all locations outlined in Appendix B using suitable survey methods and timing as per the locally relevant technical information brochures developed in Action 4.1. Additional areas may also be surveyed as opportunities arise. Animal ethics and/or survey permits are required for most – but not all – of the water mouse detection methods. If detected, effective water mouse monitoring and adaptive management programs are to be developed and implemented as per Actions 5.2, 5.3, 6.1 & 6.2. Targeted water mouse detection surveys in areas with exclusive and joint Native Title determinations are to be developed and managed, or co-developed and co-managed, by the relevant Native Title holders. | Proportion of priority detection locations surveyed. |
4.5 | Collect water mouse genetic samples. Responsibility: •Land managers and Custodians. •Ecologists. | To support research into water mouse population connectivity, non-invasive genetic samples are to be collected from a representative number of individual water mice captured at each water mouse survey location and deposited with the Australian Centre for Wildlife Genomics at the Australian Museum. Samples are to be collected in accordance with relevant permit conditions for each location. | Number of genetic samples collected. |
4.6 | Report targeted survey outcomes to the Water Mouse Recovery Team. Responsibility: •Land managers and Custodians. •Ecologists. | A short report outlining the area and habitats surveyed, methods used, and survey outcomes for all targeted water mouse detection surveys is to be made available to the Water Mouse Recovery Team within 1 year of the survey program occurring. A streamlined report template (See Action 4.2) is to be provided to land managers and Custodians, and Ecologists, to assist with this process. | Number of targeted detection survey reports received. Capacity to estimate representative EOO and AOO values. |
Targeted water mouse detection surveys may not be viable in areas with one or more of the following constraints:
1) Restrictions on access to sites of cultural significance.
2) Cultural restrictions on survey methods (e.g. night work, animal trapping).
3) Environmental restrictions on survey methods (e.g. unpredictable or excessive high tide).
4) Significant safety concerns due to remoteness and/or ruggedness.
5) Unsafe conditions due to:
a) Unmitigable risk of attack by saltwater crocodile.
b) Tidal range including storm surge and current.
c) Inclement or unpredictable weather.
Should an area be unviable for survey in the long-term, this information should be provided to the Water Mouse Recovery Team.
By implementing active search and/or remote camera methods, significant efficiencies may be achieved by adding targeted water mouse detection surveys to other activities in remote areas with the potential for habitat, and vice versa, such as:
- Sea turtle and migratory shorebird monitoring.
- Coastal habitat condition assessments.
- Flora or biocultural surveys.
- Coastline monitoring and clean-up.
- Crocodile management and egg harvesting.
- Feral animal management.
- Food and timber harvesting.
- Recreational and cultural activities such as fishing and boating.
5.2.5 Strategy 5: Incorporate water mouse into adaptive management plans to support persistence and recovery
The water mouse is an elusive species that can be easily overlooked during land management and conservation planning. To address this – in conjunction with the actions outlined for Strategy 3 (Develop communications material and implement effective partner engagement) – the water mouse is to be incorporated into an adaptive management plan or plans for every location where it is known to occur.
A water mouse adaptive management plan may be an independent plan or part of broader landscape management plans that specifically acknowledge and address potential and known local threats to the water mouse, e.g. Healthy Country Plans, Protected Area Management Plans, Catchment Management and Restoration Plans etc. Where it occurs in an area without a pre-existing or draft adaptive management plan, a short stand-alone water mouse adaptive management plan is to be developed and implemented based on a template developed by the Water Mouse Recovery Team.
For many water mouse locations, the development and implementation of an independent or broader landscape adaptive management plan for the water mouse will require the co-operation of multiple agencies with overlapping or adjacent management and/or custodianship responsibilities. It is the responsibility of these overlapping and neighbouring management and Custodianship groups to geographically define water mouse management and Custodianship areas and allocate management responsibilities appropriately to ensure effective water mouse adaptive management plans are developed and implemented.
All adaptive management plans and activities in areas with exclusive and joint Native Title determinations are to be developed and managed, or co-developed and co-managed, by the relevant Native Title holders (see Thompson et al. 2020).
The threat matrix and table in Section 4.11 and the following threat abatement and action plans provide guiding material to support the development of localised adaptive management plans for threats to the water mouse:
Table 9: Actions to support land managers and Custodians to include the water mouse in effective adaptive land management plans that identify and address local threats, and to implement these plans (Strategy 5).
Action No. | Action Description | Action Details | Performance criteria |
5.1 | Develop and disseminate a water mouse adaptive management plan template. Responsibility: Water Mouse Recovery Team. | Develop a short (approximately 3-page) water mouse adaptive management plan template for distribution (see Action 5.2 for required content). Disseminate the template, along with locally relevant information brochures (see Actions 3.2 and 4.1), to land managers and Custodians with current and historical water mouse detections on the land for which they have custodianship and who: • Are yet to develop a broad land management plan, or • Are unable to update the current broad land management plan in a timely manner. | Action completed (yes/no) |
5.2 | Water Mouse adaptive management plan development. Responsibility: Land managers and Custodians with the support of ecological consultants and the Water Mouse Recovery Team. | Ensure a water mouse adaptive management plan is in place for all locations with current and historical water mouse detections. Locations under pressure (Section 4.12) and locations in protected areas (Section 4.14) are to be prioritised. Adaptive water mouse management plans must: • Identify known and potential water mouse locations within, and adjacent to, the management area, • Identify known and potential water mouse threats and their interaction with the water mouse within the management area, • Outline management actions to reduce the impact of local threats to the water mouse and its habitat within the defined management area, • Include effective and standardised (repeatable) methods for monitoring water mouse threat trends, and • Include effective and standardised (repeatable) methods for monitoring water mouse population trends (see Actions 6.1 and 6.2). Adaptive water mouse management plans are to be reviewed at appropriate intervals depending on the water mouse trajectory and threat level. At a minimum, each adaptive management plan is to be reviewed following each water mouse population monitoring event (see Action 6.2 for monitoring intervals). Adaptive management plans may require review when significant new and relevant information about the water mouse or its threats comes to light. | Proportion of the known water mouse distribution covered by an adaptive management plan that directly addresses threats to this species. |
5.3 | Implement adaptive management plans. Responsibility: Land managers and Custodians. | Implement actions as outlined in relevant adaptive management plans to effectively reduce local threats to the water mouse. | Proportion of the known water mouse distribution where an effective adaptive management plan is implemented. |
5.4 | Report on management plan progress. Responsibility: •Water Mouse Recovery Team •Land managers and Custodians. | Provide a brief 1–2 page annual report or verbal update to the Water Mouse Recovery Team on progress against each management plan. | Proportion of management areas where threats to water mouse are managed. |
5.2.6 Strategy 6: Implement water mouse population monitoring programs
There are significant knowledge gaps about the status and population trend of the water mouse across its distribution that currently hinder effective identification and prioritisation of recovery actions. The actions outlined in Table 10 are aimed at addressing these knowledge gaps. All water mouse monitoring programs in areas with exclusive and joint Native Title determinations are to be developed and managed, or co-developed and co-managed, by the relevant Native Title holders.
Table 10: Actions to ensure effective water mouse population monitoring occurs (Strategy 6).
Action No. | Action Description | Action Details | Performance criteria |
6.1 | Develop effective water mouse monitoring plans. Responsibility: Land managers and Custodians with the support of ecological consultants and the Water Mouse Recovery Team. | As a priority, standardised and repeatable water mouse population monitoring survey plans are to be developed for all water mouse locations (see Woinarski 2018 for guidance) to assess local and national population trends, the impact of threats, and the effectiveness of management actions. Locations with historical population information (Appendix A), locations under pressure (Section 4.12) and locations in protected areas (Section 4.14) across the water mouse distribution are to be prioritised initially. Water mouse monitoring plans are to be incorporated into water mouse adaptive management plans to enable threats and population trends to be co-monitored and assessed. | Proportion of known locations where water mouse population trends are effectively monitored. |
6.2 | Implement effective water mouse monitoring programs. Responsibility: •Land managers and Custodians. •Ecologists. | Repeat standardised surveys are to be implemented within the survey intervals outlined below to assess local water mouse population trends and implement management interventions in a timely manner: • Declining: every 1–2 years. • Trend unknown, threats present: every 1–2 years. • Trend unknown, emerging threats: every 1–3 years. • Trend unknown, no threats: every 5 years. • Stable or increasing, emerging threats: every 3–5 years. • Stable or increasing, no threats: every 10 years. Genetic samples are to be collected from a representative sample of individuals at each location to support research into water mouse population connectivity (See Action 4.2). | Proportion of water mouse populations effectively monitored. National population trend estimable (yes/no). |
6.3 | Report monitoring outcomes to the Water Mouse Recovery Team. Responsibility: •Water Mouse Recovery Team •Land managers and Custodians. •Ecologists. | Provide a short report to the Water Mouse Recovery Team outlining the area and habitats surveyed, methods used, local management interventions, and survey outcomes for all water mouse monitoring surveys within 1 year of each survey program. A streamlined report template (see Action 4.2) is to be provided to land managers, Custodians, and ecologists to assist with this process. | Number of reports received. Proportion of the national water mouse population size or density and/or trend are able to be estimated. Proportion of national water mouse distribution where effects of threat management on water mouse population are discernible. |
5.2.7 Strategy 7: Water mouse research
There are significant knowledge gaps that hinder the effective identification and prioritisation of recovery actions for the water mouse. Many of these knowledge gaps will be addressed through effective national population monitoring and targeted survey programs to detect the water mouse as outlined above. However, significant gaps in knowledge about water mouse ecology, detectability, and threat impacts remain. The research questions outlined in Table 11 are aimed at addressing these knowledge gaps. Research funding is to be prioritised to address these knowledge gaps for effective water mouse management and recovery.
All water mouse research programs in areas with exclusive and joint Native Title determinations are to be developed and managed, or co-developed and co-managed, by the relevant Native Title holders.
Table 11: Research questions to address knowledge gaps about water mouse ecology and detectability, and the impact of threats to the national population (Strategy 7).
Action No. | Research Question |
7.1 | How does water mouse occurrence vary across its distribution and how is habitat best defined and mapped? |
7.2 | How resilient is the water mouse to known and potential threats, and what are the impacts of management actions? |
7.3 | How do water mouse populations respond to significant coastal disturbances including cyclone and east coast low storm surges and excessive wet season inundation? |
7.4 | What is the extent and genetic impact of water mouse fragmentation and isolation in areas with expanding coastal development, and what are the long-term prospects for water mouse viability in these areas? |
7.5 | What is the level of population connectivity across northern Australia and New Guinea, and can a genetic analysis help to determine if the water mouse occurs in undetected locations in remote northern Australia? |
7.6 | Can detection dogs, LiDAR and/or satellite imagery be used to cost-effectively increase water mouse detection probability during targeted surveys? |
7.7 | What are the drivers of intermittent water mouse detectability at locations? |
7.8 | Does water mouse ecology vary across its distribution (e.g. shelter construction and sociality, arboreal agility, biotic interactions including predation risk and diet, feeding and activity patterns, movement, breeding, life span), and how does this relate to threat susceptibility? |
7.9 | Do chemical bioaccumulation (e.g. from mosquito control) and/or microplastic pollution impact water mouse populations? |
7.10 | How can the health of water mouse populations be assessed in relation to potential threats? |
7.11 | Which standardised survey methods are suitable for monitoring water mouse population trajectories? |
The water mouse is a nationally and internationally significant species that requires interventions to reduce declines and better understand its ecology and conservation status to ensure the Commonwealth of Australia meets international obligations for biodiversity and wetland management. Significant progress in recovering the national water mouse population is likely to occur if the actions outlined in this recovery plan are comprehensively funded and implemented over the next ten years.
The cost of implementing this plan must be incorporated into the core business expenditure of partners – including funding bodies – to ensure those partners who are responsible for executing the plan can effectively collaborate, prioritise and implement actions to protect the water mouse and ensure its long-term persistence.
Table 12 outlines the action priorities, timeframes, partners, primary funding sources and costs (where estimable) required to achieve the objectives of the Water Mouse Recovery Plan. Some actions depend on other actions being completed before they can commence. These dependencies are highlighted. Other actions are non-linear and can be implemented concurrently. The timeframe for some actions is location-dependent and will vary according to the current level of local knowledge about water mouse occurrence, ecology and threats.
The detailed costs of in-situ threat management actions are unable to be quantified until three of the key actions outlined in this plan are undertaken: 1) Create a water mouse spatial habitat layer including areas supporting recovery, 2) Identify and engage all relevant partners, and 3) Develop an integrated or targeted adaptive management plan for each water mouse location to address local threats and monitor population trends. Currently, it is unclear where new actions will be required and where existing threat management programs are operating.
Costs are estimated from 2021 values for relevant activities and will likely increase with inflation over the 10-year period of the recovery plan.
Table 12: Priorities, actions, timeframes, estimated costs and primary funding sources for water mouse recovery.
Action | Priority (1-3)a | Description | Timeframeb (Dependencies) | Estimated Cost ($) | Primary Funding Source/s | Anticipated Outcome |
1.1 | Critical | Development proposals and actions are appropriately assessed and regulated. | Ongoing (Actions 2.1, 2.3) | Core Government Business | Referral/proposal fees | Remove pressure on the water mouse in locations where high human population growth drives urban and commercial expansion. |
1.2 | Critical | Propose and implement appropriate offsets to mitigate coastal development impacts. | Ongoing (Nil) | Inestimable | Referral proponents | No net decline in water mouse population due to inappropriate offsets. |
1.3 | Critical | Appropriate regulation of coastal developments. | Ongoing (Nil) | Core Government Business | Approval holder fees | Remove pressure on the water mouse in locations where high human population growth drives urban and commercial expansion. |
1.4 | Critical | Ensure a sustainable development approach to coastal development. | Ongoing (Actions 2.1, 2.3) | Inestimable | To be determined | As above |
2.1 | 1 | Map water mouse habitat at the fine scale. | Initial map completed Year 1 Reviewed every 3 years. (Action 3.1) | 20,000 10,000 per review | Commonwealth environment department. | Water Mouse habitat and areas supporting recovery are accurately depicted to support partner identification, management planning and sustainable development. |
2.2 | Critical | Identify current land managers and Custodians. | Year 1 (Action 2.1) | 10,000 | Commonwealth environment department. | Ensure all relevant land managers and Custodians are engaged in water mouse recovery. |
2.3 | Critical | Map predicted future habitat locations as sea levels rise. | Years 2–4 (Action 2.1) | 60,000 | Research institution/sc. Australian Research Council. Commonwealth environment department. | Spatial representation of likely future habitat locations and pressure points. |
2.4 | 2 | Identify future land managers and Custodians. | Year 4 (Action 2.3) | 10,000 | Commonwealth environment department. | Ensure all relevant land managers and Custodians are engaged in water mouse recovery. |
3.1 | Critical | Develop and maintain a Water Mouse Recovery Team. | Develop in Year 1, then ongoing (Nil) | 10,000 per year | Commonwealth environment department. | Effective information management and communication among partners. |
3.2 | 1 | Develop location-relevant water mouse information material. | Year 1 Reviewed every 2–3 years. (Nil) | 30,000 10,000 per review | Commonwealth environment department. | Support partner engagement and the development of effective management plans. |
3.3 | Critical | Engage landowners, managers and Custodians, and other relevant partners in water mouse recovery. | Ongoing (Actions 2.2 & 3.2) | 20,000 per year | Commonwealth environment department. | Ensure all relevant partners are engaged in water mouse recovery. |
3.4 | 1 | Raise community awareness | Ongoing (Actions 2.2 & 3.2) | Highly variable and inestimable | Commonwealth, state and local environment departments. Conservation organisations. Land managers and Custodians. | Raise the water mouse profile and increase community engagement. |
3.5 | Critical | Compile shareable water mouse recovery information in a centralised location. | Ongoing (Action 3.1) | 20,000 per year | Commonwealth environment department. | All relevant information for recovery is managed from a single point of reference. |
4.1 | 1 | Develop and disseminate locally relevant up-to-date technical information to support field survey programs. | Ongoing (Action 3.1) | 10,000 per year | Commonwealth environment department. | Increase field survey capacity and reach by empowering landowners, managers, and Custodians to implement effective non-invasive and targeted detection surveys. |
4.2 | 2 | Develop a concise water mouse survey reporting template (see Appendix C) and mechanism. | Year 1 (Action 3.1) | 5,000 | Commonwealth environment department. | Streamline reporting for landowners, managers, and Custodians, and enable data to be compiled at a single high-quality point of reference. |
4.3 | Critical | Incorporate targeted water mouse detection survey methods into general fauna surveys. | Ongoing (Actions 4.1 & 7.7) | Variable | Absorbed by survey proponents | Increased targeted survey capacity and reach to identify water mouse occurrences within the modelled distribution. |
4.4 | Critical | Implement targeted water mouse detection surveys. | Ongoing (Actions 4.1 & 7.7) | 500–10,000 per survey. Minimum 150 surveys = 75,000–1,500,000. | Land managers and Custodians. Funding grants, e.g. Caring for Country, IPA, NRM. | Increased knowledge about water mouse occurrence within the modelled distribution. |
4.5 | 1 | Collect and send water mouse genetic samples. | Ongoing (Actions 4.1, 4.3, 4.4 & 6.2) | Negligible | Land managers and Custodians. | Provide genetic information for researchers to access for population connectivity and dynamics assessments. |
4.6 | Critical | Report targeted survey outcomes to the Water Mouse Recovery Team. | Ongoing (Action 4.1) | 200–500 per report. Minimum 150 surveys = 30,000–75,000. | Land managers and Custodians | Recovery information compiled at a single point of reference. |
5.1 | Critical | Develop and disseminate a water mouse adaptive management plan template. | Year 1 (Action 3.1) | 10,000 | Commonwealth environment department. | Streamline the development of adaptive management plans for partners. |
5.2 | Critical | Develop and review water mouse adaptive management plans. | Ongoing (Actions 3.2, 3.3, 4.1 and 5.1) | 2,000–5,000 per plan. 1,500 per update. Number of plans TBD. | Land managers and Custodians. Funding grants, e.g. Caring for Country, IPA, NRM. | Land managers and Custodians clearly understand the water mouse distribution and threats on the land they manage, are able to communicate management plans among interest groups, and can adapt plans as new information comes to light. |
5.3 | Critical | Implement adaptive management plans. | Ongoing (Action 5.2) | Determined case‑by‑case | Land managers and Custodians. Funding grants, e.g. Caring for Country, IPA, NRM. | Stabilisation and reduction in threat impacts to the water mouse population across its distribution. |
5.4 | Critical | Report on adaptive management plan progress. | Ongoing (Action 5.3) | 500 per report. Number of plans TBD. | Land managers and Custodians | Recovery information compiled at a single point of reference. |
6.1 | Critical | Develop effective water mouse population monitoring plans. | Ongoing (Actions 4.1 & 7.7) | 2,000–5,000 per plan. Number of plans TBD. | Land managers and Custodians. Funding grants, e.g. Caring for Country, IPA, NRM. | Development of robust and repeatable population monitoring programs to determine population dynamics. |
6.2 | Critical | Implement effective water mouse monitoring programs. | Ongoing (Action 6.1) | 500–5,000 per survey. Number of surveys TBD. | Land managers and Custodians. Funding grants, e.g. Caring for Country, IPA, NRM. | Clear understanding about water mouse population dynamics and trends across its distribution. |
6.3 | Critical | Report monitoring outcomes to the Water Mouse Recovery Team. | Ongoing (Action 6.2) | 500 per report. Number of plans TBD. | Land managers and Custodians | Recovery information compiled at a single point of reference. |
7.1 | 1 | Research question: How does water mouse occurrence vary across its distribution and how is habitat best defined and mapped? | Years 6–7 (Actions 4.3, 4.4 & 6.2) | 30,000 | Research institution/sc. Commonwealth environment department. | Representative water mouse habitat mapping for multiple purposes including development proposals and conservation status assessment. |
7.2 | Critical | Research question: How resilient is the water mouse to known and potential threats, and what are the impacts of management actions? | Years 6–8 (Actions 4.3, 4.4, 5.4, 6.2, and 7.9) | 150,000 | Research institution/sc. | Clarity about the impact of potential threats on water mouse population dynamics and extinction risk. |
7.3 | 1 | Research question: How do water mouse populations respond to significant coastal disturbances including storm surge and excessive wet season inundation? | Years 7–9 (Actions 4.3, 4.4 & 6.2) | 130,000 | Research institution/sc. | Clarity about the impacts of inundation (e.g. short-term intensive, prolonged) on water mouse population dynamics and extinction risk. |
7.4 | Critical | Research question: What is the extent and genetic impact of water mouse fragmentation and isolation in areas with expanding coastal development, and what are the long-term prospects for water mouse viability in these areas? | Years 6–8 (Actions 2.3, 4.3, 4.4 & 6.2) | 60,000 | Research institution/sc. | Clarity about the impact of coastal development on water mouse populations and extinction risk. |
7.5 | 2 | Research question: What is the level of population connectivity across northern Australia and New Guinea, and can a genetic analysis help to determine if the water mouse occurs in undetected locations in remote northern Australia? | Years 6–8 (Actions 2.3, 4.3, 4.4 & 6.2) | 60,000 | Research institution/sc. | Clarity about water mouse distribution and evolutionary history across northern Australia in relation to current and future threats. |
7.6 | 2 | Research question: Can detection dogs, LiDAR and/or satellite imagery be used to cost-effectively increase water mouse detection probability during surveys? | Years 1–2 (Nil) | 50,000 | Research institution/sc. Commonwealth environment department. | Increased knowledge about the potential for new survey techniques to increase detection probability and significantly enhance targeted detection survey effectiveness, monitoring and research programs for this elusive species that is challenging to survey. |
7.7 | 1 | Research question: What are the drivers of intermittent water mouse detectability? | Years 1–5 (Nil) | 120,000 | Research institution/sc. | Critical information for research and monitoring programs. |
7.8 | Critical | Research question: Does water mouse ecology vary across its distribution (e.g. shelter construction and sociality, arboreal agility, feeding and activity patterns, movement, breeding), and how does this relate to threat susceptibility? | Years 4–6 (Actions 4.3 & 4.4) | 200,000 | Research institution/sc. | Significantly increased knowledge about water mouse ecology that can feed into threat impact analyses. |
7.9 | 2 | Research question: Do chemical bioaccumulation (e.g. mosquito control) and/or microplastic pollution impact water mouse populations? | Years 2–4 | 40,000 | Research institution/sc. | Clarity about the impacts of chemical and plastic pollution on water mouse. |
7.10 | 3 | Research question: How can the health of water mouse populations be assessed in relation to potential threats? | Anytime | 40,000 | Research institution/sc. | Greater insight into impacts of potential threats to the water mouse. |
7.11 | 1 | Research question: Which standardised survey methods are suitable for monitoring water mouse population trajectories? | Years 1-10 | 40,000 | Population monitoring program funding bodies (see Actions 6.1–6.3). Research institutions/sc. | Best-practice and locally-tailored national population monitoring program. |
a ‘Critical’ represents actions that are essential to the assessment and recovery of the water mouse, and values 1-3 are in decreasing order of priority with 3 being lowest priority.
b Year of this Recovery Plan. Recovery Plan commencement year = Year 1. Dependencies in black text are absolute; dependencies in grey may not be required depending on the location and/or previous engagement with water mouse recovery.
c Research Institutes, primarily through independent and Commonwealth supported research training scholarships (e.g. RTP) for postgraduate students.
There are far-reaching social and economic benefits to implementing the Water Mouse Recovery Plan. Effectively protecting and managing coastal and subcoastal wetlands for the water mouse provides co-benefits to humanity. Coastal wetlands, especially mangroves, protect coastal communities from cyclone and storm impacts and they provide breeding grounds for commercial and recreational fish and crustacean species.
Sustainable coastal development and agriculture
Considerable long-term ecological, social, and economic benefits will be achieved by – and for – all Australians by implementing the key action outlined in this recovery plan: a sustainable approach to coastal development in areas where water mouse does, or may, occur. This includes further support for protection of fish habitat areas to support fisheries industries. Perceived negative economic impacts of a sustainable approach to coastal development are likely to be due to insufficient consideration of externalities including long-term social and environmental impacts. Due consideration of all development impacts and benefits through appropriate assessment, conditioning and regulation will ensure an economically, socially and environmentally sustainable approach to coastal management occurs into the future as per the United Nations Sustainable Development Goals.
Connecting with and caring for nature
By implementing the Water Mouse Recovery Plan, Australians will be empowered to contribute to sustainably managing and better understanding nature as per Australia’s Strategy for Nature 2019-2030. This will be achieved by:
1) Connecting with nature through targeted detection surveys and long-term monitoring programs for the water mouse.
2) Caring for nature in all its diversity by addressing direct threats to water mouse persistence.
3) Sharing and building knowledge together through targeted detection and population monitoring surveys, and research into water mouse ecology and the impacts of threats.
Connection to Country and closing the gap
The Water Mouse Recovery Plan includes significant opportunities for Indigenous Peoples to lead, manage and be involved in recovery programs on Country, particularly through the Indigenous Ranger Program and National Park Joint Management initiatives. These include:
- Developing and implementing adaptive management plans for water mouse in coastal Country.
- Leading and participating in targeted water mouse detection surveys and long-term population monitoring programs.
- Developing, managing and implementing in-situ threat abatement programs for pest animals, managed stock (e.g. cattle), fire and/or weeds.
- Engaging in in-situ cultural activities during water mouse survey programs and while caring for water mouse on Country.
- Leading and participating in research programs to improve the long-term prospects for the water mouse.
Collaboration
The water mouse occurs in areas with multiple overlapping or adjacent parties that are responsible for, or have an interest in, long-term sustainable management of the land under their custodianship. The requirement within the Water Mouse Recovery Plan to develop and implement adaptive management and monitoring plans for all water mouse locations provides an additional platform for increasing management collaboration among land and sea managers and Custodians from multiple agencies and experiences.
The sustainable management of water mouse habitat and areas that support recovery benefits coastal and subcoastal ecosystems that provide protection, and fish nurseries including intertidal mangroves and saltmarshes. It also supports the stabilisation and recovery of threatened ecological communities such as paperbark (Melaleuca) and she-oak (Casuarina) wetlands, and coastal heathland (or wallum) swamps. Protection and sustainable management of these ecosystems assists with ensuring the long-term viability of threatened plants and animals that also inhabit these areas for all or part of their life cycle, such as swamp orchids (Phaius spp.), wallum frogs, flying-foxes (Pteropus spp.), and migratory shorebirds.
Effective management of invasive predators, introduced feral pigs and large herbivores, weeds and fire in coastal wetlands for the water mouse will have co-benefits for species and ecosystems that are also impacted by these threats.
This Recovery Plan will run for ten years from the time of adoption and its implementation will be managed by the Commonwealth Department of Climate Change, Energy, the Environment and Water (DCCEEW). The Water Mouse Recovery Team will be supported by DCCEEW to oversee recovery actions, compile and distribute information, and disseminate updates among partners, to assist with implementing their adaptive management plans.
Mid-term review
There will be an external review of the recovery program in the fifth year from when it was endorsed and made publicly available. The review team will assess the performance of the plan against the performance criteria outlined for each action and determine:
- If the plan continues unchanged, is varied to remove completed actions, or varied to include new conservation priorities
- If a recovery plan is no longer necessary for the species because either a Conservation Advice will suffice, or the species is removed from the threatened species list.
The Water Mouse Recovery Plan will be considered to be progressing if by 2028:
- > 95 % of coastal developments and their impact footprints within the modelled distribution of the water mouse are appropriately assessed and regulated to ensure impacts on the national water mouse population are effectively mitigated, and
- An established Water Mouse Recovery Team is effectively managing water mouse information and distributing relevant communications material among partners, and
- Known, likely and potential water mouse habitat (current and future) is accurately mapped at a fine scale using the most up-to-date ecological information, and
- > 40 % of priority water mouse detection locations have been surveyed at least once, and
- Adaptive water mouse management plans are in place and effective management actions are planned or implemented at > 80 % of water mouse locations (except locations first detected in the last 2 years), and
- An effective standardised long-term monitoring program is developed and implemented at > 80 % of water mouse locations (except locations first detected in the last 2 years), and
- > 40 % of the research questions are currently being addressed and planning for the remaining research programs is well-progressed, and
- There is a significant increase in community awareness about the water mouse across its modelled distribution, and
- There is significant leadership and participation by Indigenous Peoples in cross-collaborative planning and recovery actions for the water mouse.
Should any area/s be failing to progress, interventions will be sought to ensure progress continues on stabilising, clarifying, and recovering the national water mouse population.
Final evaluation
The recovery plan will be reviewed in 2033 and considered successful if:
- It can be demonstrated via population monitoring and approvals auditing that the water mouse population has not declined in abundance or occurrence due to coastal development, and
- Compliant adaptive management plans are in place (or under development) and management actions are effectively implemented to address threats across the water mouse distribution, and
- Knowledge about water mouse ecology and the impacts of potential threats has increased and is incorporated into adaptive management plans, and
- Up-to-date water mouse information flows freely among partners due to effective facilitation by the Water Mouse Recovery Team, and
- Inventory survey effort for water mouse has occurred at all priority locations across northern Australia (where safe and feasible to do so), and
- The ongoing decline in the national water mouse population is halted i.e. the population is demonstrated to be stable or recovering via an effective national monitoring program across its known distribution, and
- There is a significant increase in participation by Indigenous Peoples in cross-collaborative recovery planning and actions for the water mouse.
As part of the recovery plan final evaluation, the conservation status of the water mouse will be assessed against the EPBC Act species listing criteria by the Commonwealth environment department to determine if ongoing recovery interventions are required to maintain a stable population.
ABS (2021) Information Page: Regional Population. Australian Bureau of Statistics. Accessed 9 November 2021.
Allen BL, Behrendorff L, Willsher L, Kaluza J & Oakey J (2017) Recent invasion of European red foxes (Vulpes vulpes) on to Fraser Island (K’gari) and South Stradbroke Island. Austral Ecology 42, 752–758.
ASRAC (2017) Arafura Swamp Rangers Healthy Country Plan 2017–2027. Arafura Swamp Ranger Aboriginal Corporation. Ramingining, Arnhem Land.
ASRAC (2018) Media Article: New Research Looks for Rare Native Water Mouse. Arafura Swamp Ranger Aboriginal Corporation. Accessed: September 2021.
Ball D (2004) Distribution and habitat of the false water rat, Xeromys myoides Thomas, 1889 (Rodentia: Muridae) in intertidal areas of central eastern Queensland. Memoirs of the Queensland Museum 49, 487–494.
Ball D (2013) The Effect of Urban Storm-water Runoff on Sesarmid Crabs. PhD Thesis, Central Queensland University.
Ball T & Mitchell AC (2018) A new locality and range extension for the Water Mouse Xeromys myoides. North Queensland Naturalist 48, 39–45.
Banks PB & Hughes NK (2012) A review of the evidence for potential impacts of black rats (Rattus rattus) on wildlife and humans in Australia. Wildlife Research 39, 78–88.
Banks PB & Smith HM (2015) The ecological impacts of commensal species: black rats, Rattus rattus, at the urban-bushland interface. Wildlife Research 42, 86–97.
Barron Catchment Care (2017) Information Page: Rejuvenating the Barron River. Accessed: September 2021.
Bayliss P, Finlayson CM, Innes J, Norman-López A, Bartolo R, Harford A, Pettit NE, Humprey CL, van Dam R, Dutra LXC, Woodward E, Ligtermoet E, Steven A, Chariton A & Williams DK (2018) An integrated risk-assessment framework for multiple threats to floodplain values in the Kakadu Region, Australia, under a changing climate. Marine and Freshwater Research, 69, 1159–1185.
Bedrosian TA, Vaughn CA, Weil ZM & Nelson RJ (2013) Behaviour of laboratory mice is altered by light pollution within the housing environment. Animal Welfare 22, 483–487.
Beier P (2006) Effects of Artificial Night Lighting on Terrestrial Mammals. Pp 19–42 in C Rich & T Longcore (eds) Ecological Consequences of Artificial Night Lighting. Island Press, Washington.
Benfer D, Baker A, Ball T, Gynther I, Janetzki H & Fuller S (2014) Conservation genetics of the water mouse, Xeromys myoides Thomas, 1889. Australian Journal of Zoology 62, 382–392.
Bengsen AJ, West P & Krull CR (2017) Feral pigs in Australia and New Zealand: range, trend, management, and impacts of an invasive species. Pp 325–338 in M Melletti & E Meijaard (eds) Ecology, Conservation and Management of Wild Pigs and Peccaries. Cambridge University Press.
BirdLife International (2021) World Database of Key Biodiversity Areas. Developed by the KBA Partnership: BirdLife International, International Union for the Conservation of Nature, American Bird Conservancy, Amphibian Survival Alliance, Conservation International, Critical Ecosystem Partnership Fund, Global Environment Facility, Re:wild (formerly Global Wildlife Conservation), NatureServe, Rainforest Trust, Royal Society for the Protection of Birds, Wildlife Conservation Society and World Wildlife Fund. September 2021 version.
Burnett Mary Regional Group NRM (2019) Case Study: Water Mouse Conservation (1.4 MB). Burnett Mary Regional Group for Natural Resource Management. Accessed: September 2021.
Burnham M (2000) New locality records for Xeromys myoides false water-rat within the Great Sandy and Wide-Bay-Burnett management areas: results from surveys conducted February to April 1999 and March to May 2000. Queensland Parks, Maroochydore.
Chambers CL, Vojta CD, Mering ED & Davenport B (2015) Efficacy of scent‐detection dogs for locating bat roosts in trees and snags. Wildlife Society Bulletin 39, 780–787.
City of Gold Coast (2021) Water Mouse: Xeromys myoides. Draft Species Conservation Profile.
Clare JDJ, Anderson EM, MACfarland DM & Sloss BL (2015) Comparing the costs and detectability of bobcat using scat‐detecting dog and remote camera surveys in central Wisconsin. Wildlife Society Bulletin 39, 210–217.
ConocoPhillips (2020) Water Mouse Management Plan (1.5 MB). ConocoPhillips Australia Operations Ltd. Australia Pacific LNG Project. ABUE-450-EN-V01-C-00013. Accessed October 2021.
CITES (2019) Consideration of Proposals for Amendment of Appendices I and II (0.3 MB). Convention on International Trade in Endangered Species of Wild Fauna and Flora. Eighteenth meeting of the Conference of the Parties (CoP18), Colombo, Sri Lanka. Accessed: September 2021.
Dai A (2013) Increasing drought under global warming in observations and models. Nature Climate Change 3, 52–58.
DAWE (2012) Fact sheet: Australia's obligations under the Ramsar Convention: Legislative support for wetlands. Commonwealth Department of Agriculture, Water and the Environment. Accessed: December 2021.
DERM (2010) National Recovery Plan for the Water Mouse (False Water Rat) Xeromys myoides. Queensland Department of the Environment and Resource Management. Prepared for the Commonwealth Department of Sustainability, Environment, Water, Population and Communities. Accessed: October 2021.
DES (2013) Information Page: Fauna Wetland Indicator Species. Queensland Department of Environment and Science. Accessed: October 2021.
Dickman CR, Leung LK-P & Van Dyck SM (2000) Status, ecological attributes and conservation of native rodents in Queensland. Wildlife Research 27, 333–346.
Director of National Parks (2016) Kakadu National Park Management Plan 2016–2026. Kakadu National Park Board of Management. Accessed: September 2021.
Duggan JM, Heske EJ, Schooley RL, Hurt A & Whitelaw A (2011) Comparing detection dog and livetrapping surveys for a cryptic rodent. The Journal of Wildlife Management 75, 1209–1217.
Duke NC, Burrows D & Mackenzie JR (2015) Mangrove and Freshwater Wetland Habitat Status of the Torres Strait Islands: Biodiversity, Biomass and Changing Condition of Wetlands. Reef and Rainforest Research Centre Ltd, Cairns.
Duke NC, Kovacs MJ, Griffiths AD, Preece L, Hill DJE, van Oosterzee P, Mackenzie J, Morning HS & Burrows D (2017) Large-scale dieback of mangroves in Australia’s Gulf of Carpentaria: a severe ecosystem response, coincidental with an unusually extreme weather event. Marine and Freshwater Research 68, 1816–1829.
Duke NC, Mackenzie JR, Canning AD, Hutley LB, Bourke AJ, Kovacs JM, Cormier R, Staben G, Lymburner L & Ai E (2022) ENSO-driven extreme oscillations in mean sea level destabilise critical shoreline mangroves – An emerging threat. PLOS Climate 1, 0000037.
Dwyer P, Hockings M & Willmer J (1979) Mammals of Cooloola and Beerwah. Proceedings of the Royal Society of Queensland 90, 65–84.
Fell DG, Reis TR, Lynn J & Geyle MR (2018) A Terrestrial Flora and Fauna Survey of Gebar Island, Torres Strait, Queensland. David Fell Environmental for the Torres Strait Regional Authority.
Fell DG, Tom E, Tom G, Lynn J, Popic T & La Rossa C (2019) A biocultural survey at Irrki, Muralag (Prince of Wales Island). David Fell Environmental Pty Ltd.
Galaiduk R, Lebreton L, Techera E & Reisser J (2020) Transnational plastics: an Australian case for global action. Frontiers in Environmental Science 8, 115.
Gillespie GR, Brennan K, Gentles T, Hill B, Low Choy J, Mahney T, Stevens A & Stokeld D (2015). A Guide for the Use of Remote Cameras for Wildlife Survey in Northern Australia (3.7 MB). Charles Darwin University, Darwin.
Gynther IC (2011) Distribution and ecology of the water mouse Xeromys myoides on Bribie Island, south-eastern Queensland. Proceedings of the Royal Society of Queensland 117, 275–296.
Gynther IC & Janetzki H (2008) Water Mouse, Xeromys myoides. In: S Van Dyck & R Strahan (eds) The Mammals of Australia. Third Edition pp 664-666. Reed New Holland, Sydney.
Gynther IC & Janetzki H (2013) Water Mouse Xeromys myoides. In: S Van Dyck, I Gynther & A Baker (eds) Field Companion to the Mammals of Australia. Page 186. New Holland Publishers.
Harrison RL (2006) From the field: A comparison of survey methods for detecting bobcats. Wildlife Society Bulletin 34, 548–552.
Hitchcock G (1998) First record of the false water-rat, Xeromys myoides (Rodentia: Muridae), in New Guinea. Science in New Guinea 23, 141–144.
Hitchcock G (2010) Mound-and-ditch taro gardens of the Bensbach or Torassi River area, southwest Papua New Guinea. The Artefact 33, 70–90.
Hitchcock P & Gabriel J (2015) World Heritage Tentative Listed Sites in Papua New Guinea (12 MB). United Nations Educational, Scientific and Cultural Organisation (UNESCO).
Hurt A & Smith DA (2009) Conservation dogs. In Helton WS (ed.) Canine ergonomics: The science of working dogs. Pp. 175–194. CRC Press, Florida.
Kaluza J (2013) A Report on the Sunshine Coast Water Mouse Project 2012-13. Report for the Wetland Care Australia Coastal 20 Program.
Kaluza J (2016a) A report on water mouse presence along coastal wetlands of Redland Bay, Southeast Queensland. Report for Redland City Council.
Kaluza J (2016b) A report on water mouse presence along Hussey Creek south-east Queensland. Report for the EHP Investigations Unit.
Kaluza J (2016c) A report on water mouse presence at Halls Creek, Pumicestone Passage, southeast Queensland, Australia.
Kaluza J (2016d) Gold Coast City Council Water Mouse Survey 2016. WetlandCare Australia.
Kaluza J (2016e) Logan City Council Water Mouse Survey. Report for Logan City Council and WetlandCare Australia.
Kaluza J (2016f) Moreton Bay Regional Council Water Mouse Survey. Report for Moreton Bay Regional Council and Wetland Care Australia.
Kaluza J (2016g) Report findings on water mouse nest disturbance at Wangoolba Creek, Fraser Island.
Kaluza J (2016h) Threatened Species Survey for the Port of Brisbane: to determine possible water mouse presence in the port’s mangrove and saltmarsh communities.
Kaluza J (2018) The Great Sandy Strait Water Mouse Survey and Monitoring Project 2014–2018. Milestone report for the BMRG Keep the Strait Great program.
Kaluza JLD (2019) The Ecology and Conservation of the Water Mouse (Xeromys myoides) Along the Maroochy River Catchment in Southeast Queensland. Masters Thesis, The University of Queensland.
Kaluza J, Donald RL, Gynther IC, Leung LK-P & Allen BL (2016) The distribution and density of Water Mice (Xeromys myoides) in the Maroochy River of Southeast Queensland, Australia. PLoS ONE 11, e0146133.
Kaluza N & Bolzenius C (2015) Nest Distribution of Xeromys myoides on Russell Island. Report for WetlandCare Australia and Redland City Council.
Knutson TR, McBride JL, Chan J, Emanuel K, Holland G, Landsea C, Held I, Kossin JP, Srivastava AK & Sugi M (2010) Tropical cyclones and climate change. Nature Geoscience 3, 157–163.
Knutson T, Camargo SJ, Chan JCL, Emanuel K, Ho C-H, Kossin J, Mohapatra M, Satoh M, Sugi M, Walsh K & Wu L (2020) Tropical cyclones and climate change assessment: Part II: Projected response to anthropogenic warming. Bulletin of the American Meteorological Society 101, E303–E322.
Leigh KA & Dominick M (2015) An assessment of the effects of habitat structure on the scat finding performance of a wildlife detection dog. Methods in Ecology and Evolution 6, 745–752.
Long RA, Donovan TM, Mackay P, Zielinski WJ & Buzas JS (2007) Comparing scat detection dogs, cameras, and hair snares for surveying carnivores. The Journal of Wildlife Management 71, 2018–2025.
Lovelock CE, Cahoon DR, Friess DA, Guntenspergen GR, Krauss KW, Reef R, Rogers K, Saunders ML, Sidik F, Swales A, Saintilan N, Thuyen LX & Triet T (2015) The vulnerability of Indo-Pacific mangrove forests to sea-level rise. Nature 526, 559–563.
Low Choy J & Fegan M (2012) Fauna and Flora Survey of Gardangarl (Field Island) – Looking for Water Mouse, Xeromys myoides. Northern Territory Department of Land Resource Management.
Magnusson WE, Webb GJW & Taylor JA (1976) Two new locality records, a new habitat and a nest description for Xeromys myoides Thomas (Rodentia: Muridae). Australian Wildlife Research 3, 153–157.
McDougall WA (1944) An investigation of the rat pest problem in Queensland canefields: 2 species and general habits. Queensland Journal of Agricultural Science 1, 48–78.
Moise A, Abbs D, Bhend J, Chiew F, Church J, Ekstrom M, Kirono D, Lenton A, Lucas C, McInnes K, Monselesan D, Mpelasoka F, Webb L & Whetton P (2015) Climate Change in Australia Projections for Australia's NRM Regions: Monsoonal North Cluster Report. CSIRO and Bureau of Meteorology.
Morris KD (2000) The status and conservation of native rodents in Western Australia. Wildlife Research 27, 405–419.
MRC (2022) draft Sarina Beach Local Coastal Plan 2022. Mackay Regional Council and Reef Catchments.
Ovington D (1978) False water rat Xeromys myoides. Pp 94-95 in Australian Endangered Species: Mammals, Birds and Reptiles. Cassell Australia.
Paijmans K, Blake DH & Bleeker P (1971) Summary description of the Morehead-Kiunga area. In: Land Resources of the Morehead-Kiunga Area, Territory of Papua and New Guinea. Commonwealth Scientific and Industrial Research Organization (CSIRO). Part II. Land Research Series No.29.
Parker SA (1973) An annotated checklist of the native land mammals of the Northern Territory. Records of the South Australian Museum 16, 1–57.
Parker CL, Bruyere CL, Mooney PA & Lynch AH (2018) The response of land-falling tropical cyclone characteristics to projected climate change in northeast Australia. Climate Dynamics 51, 3467–3485.
Pioneer Catchment Landcare (2020). Project Summary: Raising the Profile of the local Water Mouse, Xeromys myoides. Accessed: October 2021.
QGC (2013) Environmental Management Plan: Water Mouse (Xeromys myoides) (5.6 MB). QGC Pty Ltd. Accessed: October 2021.
Queensland Government (2012) Tropical Cyclone Yasi 2011: Post Cyclone Coastal Field Investigation (4.6 MB). Department of Science, Information Technology, Innovation and the Arts. Brisbane. Accessed: October 2021.
QYAC QPWS & P (2020) Naree Budjong Djara Management Plan. Quandamooka Yoolooburrabee Aboriginal Corporation and Queensland Parks and Wildlife Service and Partnerships. Accessed October 2021.
Redhead TD & McKean JL (1975) A new record of the false water-rat, Xeromys myoides, from the Northern Territory of Australia. Australian Mammalogy 1, 347–354.
Reindl-Thompson SA, Shivik JA, Whitelaw A, Hurt A & Higgins KF (2006) Efficacy of scent dogs in detecting black-footed ferrets at a reintroduction site in South Dakota. Wildlife Society Bulletin 34, 1435–1439.
Reis TR, Fell DG, Greenhalgh A, Geyle MR, Simakal Rangers & Mura Buway Rangers (2018) A Terrestrial Fauna Survey of Dauan Island, Torres Strait, Queensland. David Fell Environmental for the Torres Strait Regional Authority Land and Sea Management Unit.
Reis TR, Fell DG, Greenhalgh A, Geyle MR & Top Western Rangers (2020) A Terrestrial Fauna Survey of Saibai Island Torres Strait, Queensland. David Fell Environmental for the Torres Strait Regional Authority Land and Sea Management Unit.
Reisser J, Shaw J, Wilcox C, Hardesty BD, Proietti M, Thums M & Pattiaratchi C (2013) Marine plastic pollution in waters around Australia: characteristics, concentrations, and pathways. PLOS ONE 8, e80466.
Roycroft E, Fabre P-H, Macdonal AJ, Moritz C, Moussalli A & Rowe KC (2022) New Guinea uplift opens ecological opportunity across a continent. Current Biology 32, 1–10.
Russell TL & Hale PT (2009) Conservation of the false water rat (Xeromys myoides) depends on landscape complementation. Australian Mammalogy 31, 81–87.
Saintilan N, Khan NS, Ashe E, Kelleway JJ, Rogers K, Woodroffe CD & Horton BP (2020) Thresholds of mangrove survival under rapid sea level rise. Science 368, 1118–1121.
SCC (2020) Annual Program Report: 2020 Coastal Fox Control Program. Sunshine Coast Council.
Setterfield SA, Rossiter-Rachor NA, Hutley LB, Douglas MM & Williams RJ (2010) Turning up the heat: the impacts of Andropogon guyanus (gamba grass) invasion on fire behaviour in northern Australian savannas. Diversity and Distributions 16, 854–861.
Shearer H (2004) Herbicides threaten North Queensland's coastal mangroves. ECOS 119, 32–33.
Sutherland G (2017) Assessing Predator Activity and Water Mouse Nest Sites at McCoys Creek Wetland Reserve. Griffith University School of Environment Industry Affiliates Program for the Gold Coast City Council.
State of Queensland (2021) K’gari (Fraser Island) Bushfire Review Report 1: 2020–21. Inspector-General Emergency Management. Accessed: October 2021.
Stobo-Wilson AM, Murphy BP, Crawford HM, Dawson SJ, Dickman CR, Doherty TS, Fleming PA, Gentle MD, Legge SM, Newsome TM, Palmer R, Rees MW, Ritchie EG, Speed J, Stuart J-M, Thompson E, Turpin J & Woinarski JCZ (2021) Sharing meals: predation on Australian mammals by the introduced European red fox compounds and complements predation by feral cats. Biological Conservation 261, 109284.
Stobo-Wilson AM, Murphy BP, Legge SM, Caceres-Escobar H, Chapple DG, Crawford HM, Dawson SJ, Dickman CR, Doherty TS, Fleming PA, Garnett ST, Gentle M, Newsome TM, Palmer R, Rees MW, Ritchie EG, Speed J, Stuart J-M, Suarez-Castro AF, Thompson E, Tulloch A, Turpin JM & Woinarski JCZ (2022) Counting the bodies: estimating the numbers and spatial variation of Australian reptiles, birds and mammals killed by two invasive mesopredators. Diversity and Distributions 28, 976–991..
Stokes VL, Banks PB, Pech RP & Spratt DM (2009) Competition in an invaded rodent community reveals black rats as a threat to native bush rats in littoral rainforest of south-eastern Australia. Journal of Applied Ecology 46, 1239–1247.
Tabari H (2020) Climate change impact on flood and extreme precipitation increases with water availability. Scientific Reports 10, 13768.
Thomas ML, Baker L, Beattie JR & Baker AM (2020) Determining the efficacy of camera traps, live capture traps, and detection dogs for locating cryptic small mammal species. Ecology and Evolution 10, 1054–1068.
Thomas O (1889) Description of a new Genus of Muridae allied to Hydromys. Proceedings of the Zoological Society of London 1889, 247–250.
Thompson K-L, Lantz TC & Ban NC (2020) A review of Indigenous knowledge and participation in environmental monitoring. Ecology and Society 25, 10.
TMR (2018) Master plan: Priority Port of Gladstone. Queensland Department of Transport and Main Roads. Accessed 6 October 2022.
Traill LM, Perhans K, Lovelock CE, Prohaska A, McFallan S, Rhodes JR & Wilson KA (2011) Managing for change: wetland transitions under sea-level rise and outcomes for threatened species. Biodiversity Research 17, 1225–1233.
Van Dyck S (1994) The Rats at Neptune’s Table. In: Australian Natural History. Autumn Edition, pp 31–37. Australian Museum.
Van Dyck S (1996) Xeromys myoides Thomas, 1889 (Rodentia: Muridae) in mangrove communities of North Stradbroke Island, southeast Queensland. Memoirs of the Queensland Museum 42, 337–366.
Van Dyck S & Durbidge E (1992) A nesting community of false water rats Xeromys myoides on the Myora sedgelands, North Stradbroke Island. Memoirs of the Queensland Museum 32, 374.
Van Dyck S & Gynther I (2003) Nesting strategies of the Water Mouse Xeromys myoides in southeast Queensland. Memoirs of the Queensland Museum 49, 453–479.
Van Dyck S & Gynther I (2012) Water Mouse Xeromys myoides Thomas, 1889. In LK Curtis, AJ Dennis, KR McDonald, PM Kyne & SJS Debus (eds). Queensland’s Threatened Animals. Pp 408–409. CSIRO, Canberra.
Van Dyck S, Janetzki H & Gynther I (2003) Artificial nesting mounds for the Water Mouse Xeromys myoides. Memoirs of the Queensland Museum 49: 480.
Van Dyck S, Janetzki H, Amey A, Sherman-Hayes L, Cox T, Frost A & Avern P (2006) Mangroves, mansions and mice – the demise of a population of water mice (Xeromys myoides) adjacent to a Gold Coast canal housing development. Australian Mammal Society Newsletter (October 2006). Page 65.
Van Dyck S, Gynther I & Baker A (2013) Field Companion to the Mammals of Australia. New Holland Publishers.
Ward M, Tulloch AI, Radford JQ, Williams BA, Reside AE, Macdonald SL, Mayfield HJ, Maron M, Possingham HP, Vine SJ, O’Connor JL, Massingham EJ, Greenville AC, Woinarski JCZ, Garnett ST, Lintermans M, Scheele BC, Carwardine J, Nimmo DG, Lindenmayer DB, Kooyman RM, Simmonds JS, Sonter LJ & Watson JEM. Nature Ecology & Evolution 4, 1321–1326.
Warfe DM, Pettit NE, Davies PM, Pusey MJ, Hamilton SK, Kennard MJ, Townsend SA, Bayliss P, Ward DP, Douglas MM, Burford MA, Finn M, Bunn SE & Halliday IA (2011) The ‘wet-dry’ in the wet-dry tropics drives river ecosystem structure and processes in northern Australia. Freshwater Biology 56, 2169–2195.
Weste G, Cahill D & Stamps DJ (1982) Mangrove dieback in north Queensland, Australia. Transactions of the British Mycological Society 79, 165–167.
White D & Power N (2016) Targeted survey and habitat assessment for the vulnerable Water Mouse (Xeromys myoides). DDWFauna Environmental Consultants for the Gold Coast City Council.
Wilson DE & Reeder DM (eds) (2005) Mammal Species of the World: A Taxonomic and Geographic Reference (3rd edition). Johns Hopkins University Press. Searchable database available at: Mammal Species of the World. Accessed: October 2021.
Woinarski JCZ (2000). The conservation status of rodents in the monsoonal tropics of the Northern Territory. Wildlife Research 27, 421–435.
Woinarski J (2004) Threatened Plants and Animals in Kakadu National Park: A Review and Recommendations for Management (6.1 MB). Northern Territory Department of Infrastructure, Planning and Environment and the Tropical Savannas Cooperative Research Centre for Parks Australia. Accessed: October 2021.
Woinarski J (2006) Threatened Species of the Northern Territory: False Water-Rat/Water Mouse Xeromys myoides. Northern Territory Department of Environment and Natural Resources. Accessed: October 2021.
Woinarski JCZ (2018) A framework for evaluating the adequacy of monitoring programs for threatened species. In: S Legge, DB Lindenmayer, NM Robinson, BC Scheele, DM Southwell & BA Wintle (eds) Monitoring threatened species and ecological communities. Pp 13–20. CSIRO Publishing, Clayton.
Woinarski JCZ & Winderlich S (2014). A Strategy for the Conservation of Threatened Species and Threatened Ecological Communities in Kakadu National Park 2014–2024. National Environmental Research Program, Northern Australia Hub, Darwin.
Woinarski J & Burbidge AA (2016). Xeromys myoides, Water Mouse. The IUCN Red list of Threatened Species. 2016: e.T23141A22454469. Accessed September 2021.
Woinarski JCA, Brennan K, Dee A, Njudumul J, Guthayguthay P & Horner P (2000) Further records of the false water-rat Xeromys myoides from coastal Northern Territory. Australian Mammalogy 21, 221–223.
Woinarski J, Brennan K, Hempel C, Armstrong M, Milne D & Chatto R (2003) Biodiversity Conservation on the Tiwi Islands, Northern Territory: Part 2. Fauna (2.9 MB). Tiwi Land Council and Northern Territory Government for the Natural Heritage Trust. Accessed: October 2021.
Woinarski JCA, Burbidge AA & Harrison PL (2014) The Action Plan for Australian Mammals 2012. CSIRO Publishing, Collingwood.
Woodroffe CD (2018) Mangrove response to sea level rise: Palaeoecological insights from macrotidal systems in northern Australia. Marine and Freshwater Research 69, 917–932.
Woodroffe CD, Rogers K, McKee KL, Mendelssohn IA & Saintilan N (2018) Mangrove sedimentation and response to relative sea-level rise. Annual Review of Marine Science 8, 243–266.
Woolley L-A, Geyle HM, Murphy BP, Legge SM, Palmer R, Dickman CR, Augusteyn J, Comer S, Doherty TS, Eager C, Edwards G, Harley DKP, Leiper I, McDonald PJ, McGregor HW, Moseby KE, Myers C, Read JL, Riley J, Stokeld D, Turpin JM & Woinarski JCZ (2019) Introduced cats (Felis catus) eating a continental fauna: inventory and traits of Australian mammal species killed. Mammal Review 49, 354–368.
Zhang F-S, Wang Y, Wu K, Xu W-Y, Wu J, Liu J-Y, Wang X-Y & Shuai L-Y (2020) Effects of artificial light at night on foraging behavior and vigilance in a nocturnal rodent. Science of the Total Environment 724, 138271.
Adkins T (2021) Personal communications by telephone and email. Statements reviewed in context and approved on 27 October 2021, prior to release for public comment. Tony Adkins has over ten years of experience managing water mouse as the Senior Conservation Officer for the Gold Coast City Council.
Ball D (2021) Personal communication by telephone and email. Statements reviewed in context and approved on 8 November 2021, prior to release for public comment. Derek Ball is a researcher with experience studying, surveying and detecting water mouse in the Mackay region.
Boody K (2021) Personal communications by telephone and email. Statements reviewed in context and approved on 27 October 2021, prior to release for public comment. Kristopher Boody is the catchment liaison officer for the Gold Coast City Council.
Duke N (2022) Personal communications by email on 28 & 29 September 2022. Dr Norman Duke is a senior research scientist with TropWater at James Cook University (JCU). He is the head of the JCU Mangrove Hub and also MangroveWatch Ltd. Norman has an intricate knowledge of mangrove ecology and dynamics, particularly across northern Australia.
Gynther I (2021) & Gynther I (2022) Unreferenced statements provided during review of the pre-release and consultation drafts of this recovery plan. Statements provided on 5 November 2021 and 20 September 2022. Ian Gynther has extensive experience surveying for water mouse along the east Queensland and northern New South Wales coasts.
HLW (2022) Submission by Healthy Land & Water in response to the draft recovery plan public consultation period. The submission included compiled feedback from representatives of Quandamooka Yoolooburrabee Aboriginal Corporation (QYAC), Southern Moreton Bay Islands Coastcare (SMBI Coastcare) and Redland City Council (RCC).
Kaluza J (2021) Personal communications by telephone and email. Statements reviewed in context and approved on 9 November 2021, prior to release for public comment. Janina Kaluza is a researcher with extensive experience studying, surveying and detecting water mouse along the southern Queensland coast.
Mitchell A (2021) Personal communication by telephone. Statements reviewed in context and approved on 29 October 2021, prior to release for public comment. Andrew Mitchell is an ecological consultant with experience surveying and detecting water mouse in the Cairns region.
SCC (2022) Submission by the Sunshine Coast Council in response to the draft recovery plan public consultation period.
Webb G (2021) Personal communication by telephone and email. Relevant statements provided on 6 Dec 2021. Graham Webb is an aquatic ecologist with the Biodiversity and Waterways section of the Sunshine Coast Council and an active member of the multi-jurisdiction Pumicestone Passage Catchment Action Plan team.
WMRG (2022) Key outcomes from expert discussions during a Teams workshop on 23 September 2022 with the Water Mouse Recovery Group. Attendees included a diversity of water mouse recovery partners from across the water mouse distribution including researchers, Natural Resource Management organisations, Indigenous Rangers, and representatives from all levels of government.
Location | Tenurea | Management Responsibility | Number of Shelter (s), Trap (t), or Individual (i) detections | Survey Year/s |
Central and southern Queensland coast |
Coomera River | Marine park, local council | Qld environment department, Gold Coast City Council | 22s 13i, 8s 17i 23i 5i 10i 0i 0s Present in mangroves | 1993–94 1997 2001 2002 2003 2004 2005 2006 After 2006 |
Browns Bay (South Stradbroke Island). | Marine park, Ramsar, conservation park. | Qld environment department, Gold Coast City Council. | 19s | 1997 |
Kirkin’s Levee (Pimpama River). | Marine park, Ramsar, local council. | Qld environment department, Gold Coast City Council. | 5i | 2004? |
Pimpama River Conservation Reserve. | Marine park, Ramsar, local council. | Qld environment department, Gold Coast City Council. | Present 4i | 2000 2012-13 |
McCoys Creek (Pimpama River). | Marine park, Ramsar, local council. | Qld environment department, Gold Coast City Council. | 11i 6s 5s, 6i | 2005 2015 2017 |
Greenfinch Reserve (Jacobs Well). | Marine park, nature refuge. | Qld environment department, Gold Coast City Council. | 1? | 1993? |
Steiglitz. | Marine park, local council. | Qld environment department, Gold Coast City Council. | 4s | 1996 |
Russell Island. | Marine park, Ramsar. | Qld environment department, Redland City Council. | 2? 8s 12s | 1996 2015 2018 |
Stingaree Island & lands adjacent to Swan Bay Restricted Access Area (Minjerribah) | ILUA, national park, marine park, Ramsar. | Quandamooka Yoolooburrabee Aboriginal Corporation, Qld environment department. | Shelters present | Pre-RAA survey (~2016) |
Stockyard (Minjerribah). | ILUA, national park, marine park, Ramsar. | Quandamooka Yoolooburrabee Aboriginal Corporation, Qld environment department. | 4i, 1s | 1992–93 |
Canalpin Creek (Minjerribah). | ILUA, national park, marine park, Ramsar. | Quandamooka Yoolooburrabee Aboriginal Corporation, Qld environment department. | 6i, 1s | 1992–93 |
Deanbilla (Minjerribah). | ILUA, national park, marine park, Ramsar. | Quandamooka Yoolooburrabee Aboriginal Corporation, Qld environment department. | 7i | 1992–93 |
Two Mile (Minjerribah). | ILUA, national park, marine park, Ramsar. | Quandamooka Yoolooburrabee Aboriginal Corporation, Qld environment department. | 1i | 1992-93 |
Myora Springs (Minjerribah). | Exclusive native title, ILUA, national park, marine mark, Ramsar, conservation park. | Quandamooka Yoolooburrabee Aboriginal Corporation, Qld environment department, Redland City Council. | 1i 6i 7i | 1978 1992-93 1992 |
Rainbow Channel (Minjerribah). | ILUA, marine park, Ramsar. | Quandamooka Yoolooburrabee Aboriginal Corporation, Qld environment department. | 80i, 10s | 1992–93 |
Chiggil Chiggil/ Amity (Minjerribah). | Exclusive native title, national park, marine park, Ramsar. | Quandamooka Yoolooburrabee Aboriginal Corporation, Qld environment department. | 7i, 4s | 1992-93 |
Beachmere (Caboolture River). | Marine park, Ramsar, local council. | Qld environment department, Moreton Bay Regional Council. | 1s | 2015 |
White Patch (Bribie Island west). | National park, marine park, Ramsar. | Qld environment department. | 1s | 1996 |
Gallagher Point (Bribie Island west). | National park, marine park, Ramsar. | Qld environment department. | 5s 6s | 1999 2009 |
Third Lagoon (Bribie Island east). | National park, marine park, Ramsar. | Qld environment department. | 1i 1i 0i | 1984 1985 1991-96 |
Second Lagoon (Bribie Island east). | National park, marine park, Ramsar. | Qld environment department. | 2i | 2010 |
Meldale. | Marine park, Ramsar, unallocated state land. | Qld environment department. | 3s | 2022 |
Donnybrook. | Marine park, Ramsar, local council. | Qld environment department, Moreton Bay Regional Council. | 17s | 1996 |
Bullock Creek CP (Pumicestone Passage). | Marine park, Ramsar, conservation park. | Qld environment department. | 3s | 1996 |
North of Bullock Creek CP (Pumicestone Passage). | National park, marine park, Ramsar. | Qld environment department. | 8s 1 | 1996 2011 |
Glass Mountain Creek (Pumicestone Passage). | Marine park, Ramsar. | Qld environment department. | 2 51s | 1994 2012 |
Halls Creek (Pumicestone Passage). | National park, marine park, Ramsar. | Qld environment department. | 18s | 2013 |
Bells Creek (Pumicestone Passage). | Ramsar, state forest, local council. | Qld environment department, Qld forestry department, Sunshine Coast Council. | 2s | 2012 |
Coochin Creek (Pumicestone Passage). | Marine park, Ramsar. | Qld environment department. | 1t | 1994 |
Hussey Creek (Pumicestone Passage). | Marine park, Ramsar. | Qld environment department. | 19s | 2012 |
Subset of Hussey Creek. | Marine park, Ramsar. | Qld environment department. | 5s 2s | 2012 2016 |
Beerwah Scientific Area 1. | National park, forest reserve. | Qld environment department, Qld forestry department. | 1 1i 1i | 1966 1975 2000 |
Mooloolah River. | National park. | Qld environment department. | 5s | 2013 |
Maroochy River. | Fish habitat area, conservation park, nature refuge, private property. | Qld environment department, Sunshine Coast Council. | 1s 185s Present | 1996 2011–15 2021 |
Noosa River North Shore. | Fish habitat area, national park, local council. | Qld environment department, Noosa Council. | 1i 7s | 1975 1997 |
Kinaba Island (Lake Cootharaba). | Fish habitat area, national park, local council. | Qld environment department, Noosa Council. | 1 1 | 1975 1976 |
Noosa Plain – north. | National park | Qld environment department. | Present Present Present | 1970 1975 2003 |
Noosa Plain – south. | National park, fauna reserve. | Qld environment department. | Present | 1975 |
Cooloola Cove (Great Sandy Strait). | Marine park, Ramsar, national park. | Qld environment department. | 1 | 1997 |
Poverty Point (Great Sandy Strait). | Marine park, Ramsar, national park. | Qld environment department. | 8s | 2000 |
Cooloola Creek (Great Sandy Strait). | Marine park, Ramsar, national park. | Qld environment department. | 4s | 2000 |
Seary’s Creek (Great Sandy Strait). | Marine park, Ramsar, national park. | Qld environment department. | 18s | 2000 |
Carlo Creek (Great Sandy Strait). | Marine park, Ramsar, national park. | Qld environment department. | 8s | 2000 |
Carlo Point (Great Sandy Strait). | Marine Park, Ramsar, Non-exclusive Native Title. | Qld environment department, Butchulla Aboriginal Corporation. | 3s 0s | 2000 2014–16 |
Inskip & Bullock Creek (Great Sandy Strait). | Marine park, Ramsar, Non-exclusive native title. | Qld environment department, Butchulla Aboriginal Corporation. | 4s 2s 4s | 2000 2006 2015–17 |
Tin Can Bay (Great Sandy Strait). | Marine park, Ramsar, Non-exclusive native title. | Qld environment department, Butchulla Aboriginal Corporation, Commonwealth Defence Department. | 1i | ? |
Camp Kerr (Great Sandy Strait). | Marine park, Ramsar, Non-exclusive native title, defence. | Qld environment department, Butchulla Aboriginal Corporation, Commonwealth defence department. | 7s | 2015–17 |
Snapper Creek (Great Sandy Strait). | Defence. | Commonwealth defence department. | 2s | 2015-17 |
Boronia (Great Sandy Strait). | Marine park, Ramsar, Non-exclusive native title, defence. | Qld environment department, Butchulla Aboriginal Corporation, Commonwealth defence department. | 10s | 2000 |
Teebar Creek (Great Sandy Strait). | Marine park, Ramsar, Non-exclusive native title, defence. | Qld environment department, Butchulla Aboriginal Corporation, Commonwealth defence department. | 34s | 2000 |
Little Stoney Creek (Great Sandy Strait). | Marine park, Ramsar, Non-exclusive native title, defence. | Qld environment department, Butchulla Aboriginal Corporation, Commonwealth defence department. | 9s | 2000 |
Kauri Creek (Great Sandy Strait). | Marine park, Ramsar, Non-exclusive native title, defence. | Qld environment department, Butchulla Aboriginal Corporation, Commonwealth defence department. | 6s 29s 33s | 1997 2000 2015–17 |
Mosquito Creek (Great Sandy Strait). | Marine park, Ramsar, forest reserve, non-exclusive native title. | Qld environment department, Qld forestry department, Butchulla Aboriginal Corporation. | 12s | 2000 |
Great Sandy Conservation Park – Kauri Creek mouth (Great Sandy Strait). | Exclusive native title, marine park, Ramsar, conservation park. | Butchulla Aboriginal Corporation, Qld environment department. | 13s | 2000 |
Cowra Point (Great Sandy Strait). | Exclusive native title, marine park, Ramsar, conservation park. | Butchulla Aboriginal Corporation, Qld environment department. | 8s | 2000 |
Buttha Creek (Great Sandy Strait) | Exclusive native title, marine park, Ramsar, forest reserve. | Qld environment department, Qld forestry department, Butchulla Aboriginal Corporation. | 1s | 2000 |
Poona Creek (Great Sandy Strait). | Marine park, Ramsar, forest reserve, non-exclusive native title. | Qld environment department, Qld forestry department, Butchulla Aboriginal Corporation. | 1i, 2s | 1999 |
Big Tuan Creek (Great Sandy Strait). | Marine park, Ramsar, forest reserve, non-exclusive native title. | Qld environment department, Qld forestry department, Butchulla Aboriginal Corporation. | 8s | 2000 |
Raven Hill (Great Sandy Strait). | Exclusive native title, marine park, Ramsar, national park. | Butchulla Aboriginal Corporation, Qld environment department. | 4s | 2015-17 |
Kalah Creek (Great Sandy Strait). | Marine park, Ramsar, national park, non-exclusive native title. | Qld environment department, Butchulla Aboriginal Corporation. | 5s | 2000 |
Tandora (Great Sandy Strait). | Marine park, Ramsar, private property, non-exclusive native title. | Qld environment department, Tandora Grazing Pty Ltd, Butchulla Aboriginal Corporation. | 23s | 2015–17 |
River Heads (Great Sandy Strait). | Marine park, Ramsar, non-exclusive native title. | Qld environment department, Butchulla Aboriginal Corporation. | 3s | 2015-17 |
Booral. | Local council. | Fraser Coast Regional Council, Butchulla Aboriginal Corporation | 5s 0s | 2000 2015–17 |
August/Talli Creek (K’gari, Great Sandy Strait). | Marine park, Ramsar, national park, ILUA. | Butchulla Aboriginal Corporation, Qld environment department. | 6s | 2000 |
Dream Island/ Garry’s Anchorage (K’gari, Great Sandy Strait). | Marine park, Ramsar, national park, ILUA. | Butchulla Aboriginal Corporation, Qld environment department. | 5s | 2000 |
Wangoolba Creek (K’gari, Great Sandy Strait). | Marine park, Ramsar, national park, ILUA. | Butchulla Aboriginal Corporation, Qld environment department. | 8s 1i | 2015-17 2019 |
Dudonga/Kingfisher Bay (K’gari, Great Sandy Strait). | Marine park, Ramsar, national park, ILUA. | Butchulla Aboriginal Corporation, Qld environment department. | 3i | 1997 |
Moon Point (K’gari, Great Sandy Strait). | Marine park, Ramsar, national park, ILUA. | Butchulla Aboriginal Corporation, Qld environment department. | 1? | 1997 |
O’Reagan’s Creek. | Marine park, conservation park, non-exclusive native title. | Qld Environment Department, Butchulla Aboriginal Corporation. | 7s | 2015-17 |
Burrum Heads. | Marine park, national park, non-exclusive native title, local council. | Qld environment department, Butchulla Aboriginal Corporation, Bundaberg Regional Council. | 11s | 2015-17 |
Elliot Heads/Turtle Cove. | Marine park, ILUA. | Qld environment department, First Nations Bailai, Gurang, Gooreng Gooreng, Taribelang Bunda People Aboriginal Corporation. | 4s | 2015-17 |
Kolan River/Moore Park. | Marine park, conservation park, ILUA. | Qld environment department, North Burnett Regional Council, First Nations Bailai, Gurang, Gooreng Gooreng, Taribelang Bunda People Aboriginal Corporation. | 4s | 2015-17 |
Eurimbula National Park. | Marine park, national park, non-exclusive native title. | Qld environment department, First Nations Bailai, Gurang, Gooreng Gooreng, Taribelang Bunda People Aboriginal Corporation. | 3s 2s | 2000 2015-17 |
Roundhill Creek (Agnes Waters). | National park, local council, ILUA. | Qld environment department, Gladstone Regional Council, First Nations Bailai, Gurang, Gooreng Gooreng, Taribelang Bunda People Aboriginal Corporation. | 13s 1 | 2000 2011 |
Bustard Bay. | High conservation value aquatic ecosystem, private property. | Qld environment department, Private land owners. | Robust population | 2013 |
Middle Creek (Bustard Bay). | Marine park, national park, resources reserve, ILUA. | Qld environment department, Qld Resources Department, First Nations Bailai, Gurang, Gooreng Gooreng, Taribelang Bunda People Aboriginal Corporation. | 7s | 2000 |
Middle Island Causeway (Bustard Bay). | Marine park, ILUA. | Qld environment department, First Nations Bailai, Gurang, Gooreng Gooreng, Taribelang Bunda People Aboriginal Corporation. | 1? | 2011 |
Mort Creek. | Marine park, national park, ILUA. | Qld environment department, Gladstone Regional Council, First Nations Bailai, Gurang, Gooreng Gooreng, Taribelang Bunda People Aboriginal Corporation. | 2s | 2000 |
Turkey Beach Foreshore. | Marine park, local council, ILUA. | Qld environment department, Gladstone Regional Council, Gladstone Regional Council, First Nations Bailai, Gurang, Gooreng Gooreng, Taribelang Bunda People Aboriginal Corporation. | 4s | 2000 |
Targinie and Humpy Creek (Gladstone Harbour). | Private property, ILUA. | Gladstone Ports Corporation, First Nations Bailai, Gurang, Gooreng Gooreng, Taribelang Bunda People Aboriginal Corporation. | 6i ? | 2011 2013 |
Facing Island. | ILUA. | Gladstone Ports Corporation, First Nations Bailai, Gurang, Gooreng Gooreng, Taribelang Bunda People Aboriginal Corporation. | 3? | 1998 |
Laird Point (Curtis Island). | Private property, ILUA. | Australia Pacific LNG, First Nations Bailai, Gurang, Gooreng Gooreng, Taribelang Bunda People Aboriginal Corporation. | 0 1i 0 0 0 | 2010 2011 2014 2015 2019 |
Cape Palmerston. | Marine park, national park, non-exclusive native title. | Qld environment department, Yuwi Aboriginal Corporation. | 1i 5 Present | 1999 2011 2019 |
Freshwater Point (Sarina). | Marine park, non-exclusive native title. | Qld environment department, Mackay Regional Council, Yuwi Aboriginal Corporation. | 10i 2s 1s | 1999 2008 2011 |
Sandringham Bay. | Conservation park, exclusive native title. | Qld environment department, Yuwi Aboriginal Corporation. | 2i 14s 9s Present | 1999 2011 2012 2019 |
Bakers Creek. | Conservation park, exclusive native title. | Qld environment department, Yuwi Aboriginal Corporation. | 1s 0 | 2000 2019 |
19 km from Mackay, 1 mile from the sea. | Unknown. | Unknown. | 5i | 1944 |
Bucasia/Eimeo. | Private property, non-exclusive native title. | Mackay Regional Council, Yuwi Aboriginal Corporation. | 1i 2i 2s | 1939 1999 2011 |
Cape Hillsborough. | Marine park, national park, non-exclusive native title. | Qld environment department, Yuwi Aboriginal Corporation. | 1s Present | 2013 2019 |
Skull Knob. | Marine park, exclusive & non-exclusive native title, conservation park. | Qld environment department, Yuwi Aboriginal Corporation. | Present | 2019 |
Goorganga Plains. | Marine park. | Qld environment department. | 2? | 1982 |
Flying Fox Island (Proserpine River). | Marine park, unallocated state land. | Qld resources department, Whitsunday Regional Council. | 1i | 1999 |
Pioneer Bay, Waite Creek (Cannonvale). | Marine park, unallocated state land. | Qld resources department, Whitsunday Regional Council. | 1i | 1999 |
Far North Queensland |
Hinchinbrook channel | | | Present | 2000 |
Inarlinga | Defence. | Commonwealth defence department. | Present | 1993 |
Cairns Airport. | Private property. | North Queensland Airports Group. | 1i | 2017 |
Redden Island (Cairns). | Private property. | North Queensland Airports Group. | Feeding sign | 2017 |
Barr Creek (Cairns). | Conservation reserve. | Cairns City Council. | 3t | 2017 |
Richter Creek (Cairns). | Private property, conservation reserve. | Cairns City Council. | Feeding sign | 2017 |
Top End, Northern Territory |
Tomkinson River (8 km from mouth). | Indigenous protected area. | Djelk Rangers. | 4i 3 or 4i | Prior to 1975 1975 |
Tomkinson River (53 km from mouth). | Indigenous protected area. | Djelk Rangers. | 1i | 1975 |
Glyde River. | Aboriginal freehold. | Arafura Swamp Rangers Aboriginal Corporation. | 2i 1i | 1998 1999 |
South Alligator River. | National park, Ramsar. | Kakadu Board of Management, Parks Australia. | 1i | 1903 |
Melville Island (Tiwi Islands). | Local council. | Tiwi Islands Land and Sea Management, Tiwi Islands Council. | 3i | 1975 |
Daly River. | Private lease. | Stapleton Station. | 2i | 1972 |
Papua New Guinea |
Wando Village. | Private property, Ramsar. | Wartha People. | 2i | 1998 |
a ILUA = Indigenous Land Use Agreement
Priority locations include:
- Areas with extensive mangrove, saltmarsh and/or shallow subcoastal freshwater wetlands where the water mouse has not been detected or confirmed, but may occur.
- Areas with potential for habitat that have not been surveyed and are near locations where the water mouse is abundant.
- Remote areas where the water mouse has not been detected for more than 20 years due to no or limited survey effort.
- Freshwater areas with historical records and/or with potential for habitat that are accessible and could be suitable for investigating water mouse ecology and threats in freshwater habitat.
Region | Location |
Australia | Locations with unverified detections e.g. Shoalwater Bay. |
New South Wales | Bundjalung National Park. Brunswick Heads and Tyagarah Nature Reserve. Tweed River and Richmond River systems. |
South and central Queensland coasts | The islands and coastal areas of Moreton Bay, particularly: - From Coochiemudlo Island south to the Coomera River.
- Tingalpa Creek.
- Crab Island and adjacent coastal areas of Kooringal on Mulgumpin/Moreton Island
- Port of Brisbane, Green Island, Mud Island, and from Boondall Wetlands to the Brisbane airport.
- Hays Inlet and the Pine River mouth.
- Lower Caboolture River and Burpengary Creek.
Northern Pumicestone Passage. Mooloolah River from the estuary to Ewen Maddock Dam. Intermittently closed and open lakes and lagoons of the Sunshine Coast including Tooway Creek, Coondibah Creek, Currimundi Lake and Stumers Creek. Upper Noosa River wetlands (Cooloola National Park, Lake Cootharaba, Lake Cooroibah) and Lake Weyba. Burrum-Isis Conservation Reserve, Burrum Coast National Park, and Barubbra Island Conservation Reserve. Coastal wetlands of Deepwater National Park, Baffle Creek and the Kolan River Conservation Reserve. Northwest Curtis Island and the Fitzroy River mouth. Cawarral Creek and Corio Bay. Shoalwater Bay. Port of St Lawrence from Herbert Creek to Allandale Island and the Clairview Island area. Estuaries around West Hill Island including north to Gillinbin Creek. Estuaries around Freshwater Point: Rocky Creek, Tommy Creek, Plane Creek. Proserpine River. Whitsunday Island. Mouth of the Gregory River (south of Bowen). |
Northern Queensland coast | Coastal wetland systems from Cungulla to Bowen. Bohle River Conservation reserve and Cleveland Bay (Townsville). Extensive mangrove systems of the Hinchinbrook channel. Coastal wetlands from Cardwell to Cairns including Edmund Kennedy National Park and the Hull River, Moresby River, and the Russell and Mulgrave Rivers. Trinity Inlet and the Lower Barron River. Coastal wetland systems from Port Douglas to Mossman. Lower reaches of the Daintree River. Endeavour and Annan Rivers (Cooktown). Mangrove systems around the Starcke River mouth, Cape Flattery. Coastal wetlands of Rinyirru/Lakefield National Park. Lower reaches of the Stewart River and Silver Plains. Lower reaches of the Lockhart River. Harmer Creek, Shelburne Bay. Jackey Jackey Creek and the lower Escape River coastal wetlands. Saibai, Boigu, Badu, Moa, Ngarupai and Muralag Islands (Torres Strait). Lower reaches of the Wenlock and Ducie Rivers. Coastal wetlands from Cullen Point to Pine River Bay. Mission River and Hey River. Lower reaches of the Watson and Archer Rivers and coastal wetlands systems south to Walngal. Halroyd River delta including Kendall River and Christmas Creek. Extensive coastal wetlands around Kowanyama and the Mitchell River. Staaten River and Gilbert River delta floodplains. Norman, Bynoe and Flinders River coastal floodplains. Leichhardt, Albert and Nicholson River coastal floodplains. Low coastal floodplains from Moonlight Creek to Settlement Creek including Mornington Island. |
Top End, NT | Coastal wetlands around the mouth of the Calvert River. Coastal mangroves and wetlands on and adjacent to the Pellew Islands. Mouth of the Limmen Bight and Roper Rivers. Coastal lake systems of Groote Eylandt. Lower reaches of the river systems in Blue Mud Bay. Coastal mangroves in Buckingham and Arnhem Bays. Hutchinson Strait and Woolen River. Extensive coastal wetlands on and adjacent to the Crocodile Islands. Arafura Swamp and the upper reaches of Gulbawangay and Goyder Rivers. Liverpool and Blyth Rivers and floodplains. King River mangroves and Goomadeer River floodplains. Ilamaryi River to Myrgenella Creek. The extensive coastal wetlands and floodplains of Adelaide River, Mary River, South Alligator River and East Alligator River. Wetland systems of the Tiwi Islands and Clarence Strait. Port Darwin and Hope Inlet. Extensive coastal wetlands from Bynoe Harbour to Wadeye, including Daly River. New Moon Inlet to the Keep River. |
Kimberley, WA | Cambridge Gulf. Prince Frederick Harbour, the St George Basin and lower Glenelg River. Walcott Inlet and Secure Bay. Mangrove and freshwater wetlands north of the Robinson River mouth. Mangrove-lined inlets of the western Dampier Peninsula and Roebuck Bay. |
The following report framework provides guidance for water mouse recovery partners about how to record and share information from targeted surveys for the water mouse, and incidental detections, in a way that will support recovery actions for this species.
Recovery partners are encouraged to share either the full report (or a redacted version where sensitivities occur) among recovery colleagues to support collaboration. Recovery partners are also recommended to submit full or redacted reports when requested for the 5-year and 10-year reviews of this recovery plan.
The style of this template is minimally prescriptive to allow recovery partners to tailor it to suit their needs. The use of individual style, photos, and narrative (stories) to support the report is encouraged.
Report section | Key elements | Example/s |
Title | Primary outcome and location. | “No water mouse detected during targeted camera surveys in the Proserpine River area, Qld.” “Suspected water mouse shelter in St George Basin, WA.” “Water mouse re-detected in the Arafura Swamp area of the NT.” “2023 survey of the water mouse population at Maroochy River Wetland Sanctuary, Qld.” |
Author details | Name/s, organisation, long-term email contact. | “Joe Bloggs, abc Rangers, rangers@abc.com.au” |
Partner organisation details | Name, role in the program. | “Implemented in partnership with xyz partner group with funding from jkl organisation.” |
Survey details | Define the boundaries of the survey site with reference to obvious landmarks. | “Mangrove and supratidal areas on both sides of the Richmond River from the Burns Point Ferry downstream to the Missingham Bridge, Lighthouse Beach, and South Ballina Beach.” |
Technical or hand-marked survey site map with a satellite image base layer. | 
|
Describe the area with reference to vegetation, salinity, waves, tides, floods etc. | “Mangrove forests (see photos) dominated by stilted mangroves (Rhizophora sp.) and yellow mangroves (Ceriops tagal), and adjacent saltmarsh. Tidal range is approximately 7 m in this sheltered bay. There is little wave action except during cyclones and severe storms. “ “Subcoastal swamps with fresh to slightly brackish water that are dominated by paperbarks (Melaleuca sp.) (see photos). The water is still and permanent, but floods extensively in the wet season.” “Refer to (report, paper, document) for more detailed site information.” |
Describe any confirmed or potential threats (see Section 4) in or near the area that was surveyed or explored. | “Foxes are regularly spotted around the wetland, and cats probably also move across the area. The site is bound by urban development with concentrated storm water runoff, potential chemical pollution from mosquito spraying, and high levels of boating. There is no vehicle access to the site.” “There are no obvious threats, but there are regular cyclones and storms.” “Pigs are common in this region and there were digs throughout the area.” |
Provide details about the survey or exploration technique/s that were used, including dates. Discuss any previous survey efforts at the site or in the area. | “We initially walked through the area to search for signs of water mouse, then set out cameras in areas with potential shelters and feeding sign (see photos). Two cameras, baited with gar fish, were set at each of 12 potential sites (see map & photos). All the cameras were set on Friday 11th August and collected on Monday 14th August (2023) before the big tides returned.” “We re-surveyed the same area as (organisation) in (date) (report reference).” “We were collecting mud crabs and took a few photos of suspected shelters and feeding sign (see photos).” “We surveyed all upper tidal and supratidal areas with a detection dog.” “We were exploring and found a dead water mouse at (GPS point).” |
Technical or hand-marked map showing where each survey method was used. May be combined with the site map. | 
|
Outcomes & learnings | State if there were any suspected or confirmed detections of water mouse. Provide details, including GPS points, and photos where available, for all detections. | “We didn’t find any signs of water mouse.” “The detection dog indicated that there was water mouse scent in the upper mangrove zone throughout the surveyed area.” “The cameras at sites 2, 6 and 9 (see map) photographed what appear to be water mouse (see photos). The GPS coordinates for these points are…” “Photos of the suspected shelter (see images and map) were confirmed by species expert (name) as likely to have been created by water mouse. The approximate GPS coordinate of this site is…” “Four of the eight suspected shelters (see map) showed signs of water mouse activity – fresh mud daubing, a strong acrid odour and/or likely feeding sign nearby. The GPS points for these locations are…” “Of the 36 shelters that were detected, 13 known shelters showed signs of recent activity, 9 known shelters did not show signs of recent activity, and 14 new shelters require follow-up to confirm they are inhabited by the water mouse, with 11 of these showing signs of recent activity (see map, photos, table of GPS locations).” "A female water mouse was trapped at (GPS location) on (date) and a tissue sample was collected for genetic analysis. After consulting with xxx Museum, the tissue sample and photos of the water mouse were sent to yyy.” |
Follow-up actions | Brief statement about future activities at the site or in the region for water mouse. | “We plan to continue searching for water mouse in the region.” “We plan to deploy cameras at the suspected site.” “We plan to expand the survey program to better understand where the water mouse occurs across the site, and how abundant it is in this new area.” “We plan to continue monitoring the water mouse in this area every year.” “We will consider this new information during our pig, fire and weed management programs.” “No follow-up action planned at this stage.” |
For assistance with confirming water mouse shelters, feeding sign, or individuals from photos, contact your State or Territory Museum or environment department, or a water mouse recovery partner who has agreed to provide this service.












































































