AAD | Australian Antarctic Division of the Australian Government Department of Climate Change, Energy, the Environment and Water. |
AAT | Australian Antarctic Territory. |
ACAP | Agreement on the Conservation of Albatrosses and Petrels, done 19 June 2001, 2258 UNTS 257 (entered into force 1 February 2004). |
AFMA | Australian Fisheries Management Authority. |
ANARE | Australian National Antarctic Research Expeditions. |
Australian Fishing Zone | Area of waters between three nautical miles and 200 nautical miles seaward of the baselines. |
Australia's jurisdiction | The area over which Australia has the power to make legal decisions and judgements. |
ATEP Act | Antarctic Treaty Environment Protection Act 1980 (Cth). |
Bycatch | Unintentional catch of a seabird during fishing. |
Caught | Where a seabird is either hooked, entangled or struck by fishing gear, regardless of whether the seabird is landed on board the fishing vessel. |
CBD | Convention on Biological Diversity. |
CMS | Convention on the Conservation of Migratory Species of Wild Animals, done 23 June 1979, 1651 UNTS 333 (entered into force 1 November 1983). |
DCCEEW | Australian Government Department of Climate Change, Energy, the Environment and Water. |
EEZ | Exclusive Economic Zone—area of waters between 12 nautical miles and 200 nautical miles seaward of the baselines. |
Electronic monitoring system | Video recording system involving cameras positioned on a fishing vessel enabling fishing operations (including setting and hauling) to be recorded, and viewed later by regulatory authorities for management purposes. |
EPBC Act | Environment Protection and Biodiversity Conservation Act 1999 (Cth). |
FAO | Food and Agriculture Organization of the United Nations. |
Fishing gear | Any fishing gear deployed by a fishing vessel including seabird mitigation devices and technologies. |
Fishing operator | Legal or natural person who holds a fishing concession, as defined under fisheries legislation: for example, the Fisheries Management Act 1991 (Cth). |
Gillnet fishing | Gillnets are long rectangular nets, which are set horizontally along the ocean floor. The nets are kept vertical by floats along the top and weights along the bottom. Fish swim into the net and are entangled by the gills, fins and spines. |
Harmful marine debris | All plastics and other types of debris from domestic or international sources that may cause harm to vertebrate marine wildlife. This includes land-sourced plastic garbage (for example, bags, bottles, ropes, fibreglass, piping, insulation, paints and adhesives), derelict fishing gear from recreational and commercial fishing activities, and ship-sourced, solid non-biodegradable floating materials lost or disposed of at sea. |
HIMI | External Territory of Heard Island and McDonald Islands. |
Impact | A marked effect or influence. |
Independent monitoring | Monitoring of fishing operations by regulatory authorities using a scientific observer or other independent observer, and/or an electronic monitoring system. |
Interaction | In the context of this recovery plan an interaction with a seabird occurs where a seabird is observed as caught under one of the following situations: - Dead not landed on board—birds observed to be killed by direct interaction with fishing gear, but not landed on the fishing vessel.
- Dead landed on board – birds killed by direct interaction with fishing gear and landed on the fishing vessel.
- Alive landed on board the fishing vessel following direct interaction with fishing gear:
a) injured, or b) released uninjured. 4. Alive and released while not on board the fishing vessel following direct interaction with fishing gear: a) injured, or b) released uninjured. |
IUCN | International Union for Conservation of Nature. |
KBA | Key Biodiversity Areas. |
Longline fishing | Setting and hauling of one or more single lines (mainline) that contains many individual hooks on branch lines. The mainline can either be anchored or drifting. It can be oriented vertically or horizontally, and vary considerably in length and number of hooks. Longline fishing includes using any configuration of a pelagic or demersal longline. |
NGO | Non-government organisation. |
Offal | Discarded waste from the processing of fish (including, among other things, discarded fish and other organisms, and used baits). |
Pelagic finfish | Fish that live in the upper layers of the sea or ocean. |
Purse seine | A generally small-meshed net with a floating top and weighted bottom that is deployed to encircle target species. After encirclement, a wire threaded through the bottom of the net is pulled to tighten the net like a purse, enclosing the catch within and allowing the net to be brought to the fishing vessel where the catch is retrieved either by brailing, pumping or bringing the net and catch on board the vessel. |
Precautionary approach | The precautionary approach is included as one of the principles of ecologically sustainable development in subsection 3A(a) of the EPBC Act, as follows: 'if there are threats of serious or irreversible environmental damage, a lack of full scientific certainty should not be used as a reason for postponing measures to prevent environmental degradation'. |
Range States | Any State that exercises jurisdiction over any part of the range of albatross or petrels, or a State, flag vessels of which are engaged outside of its national jurisdictional limits in taking, or have the potential to take, albatrosses and petrels. |
Recovery plan | A plan setting out the research and management actions necessary to stop the decline of, and support the recovery of, listed threatened species or threatened ecological communities under the EPBC Act. |
Seabird | A bird that frequents the sea or coast. For the purposes of this recovery plan, a seabird includes all species in the Class Aves. |
Seabird mitigation device | Device designed to reduce the likelihood of interactions between seabirds and fishing gear. |
Recovery Team | Forum established by the department to discuss implementation and effectiveness of provisions of this recovery plan. Participation includes representatives from Australian and state governments, the fishing industry, non-governmental organisations, academic institutions, scientists, innovators, manufacturers, and others with an interest and expertise in conserving threatened albatrosses and petrels. |
Threatening process | A process that threatens, or may threaten, the survival, abundance or evolutionary development of a native species or ecological community: see subsection 188(3) of the EPBC Act. |
Threat abatement plan | A plan providing for the research, management, and any other actions necessary to reduce the impact of a listed key threatening process on impacted species and ecological communities. |
Trawl fishing | Pulling a fishing net through the water behind one or more boats. Trawl fishing includes using any configuration of a pelagic or demersal trawl net. |
TSSC | Threatened Species Scientific Committee. |
The National Recovery Plan for albatrosses and petrels (2022) provides a national strategy to guide the activities of government, industry, research organisations, and other stakeholders in the protection, conservation and management of listed threatened albatross and petrel species. The plan outlines the research and management actions necessary to stop the decline and support the recovery of the species so that their chances of long-term survival in nature are maximised. The plan also outlines the major benefits to other albatross and petrel species that are not listed as threatened, but will be affected by the implementation of the plan.
This recovery plan is the third plan for threatened albatross and petrel species under the Environment Protection and Biodiversity Conservation Act 1999 (Cth) (EPBC Act). The plan replaces the previous plan adopted in 2011.
Seabirds remain among the world's most threatened bird species. Albatrosses and petrels in particular face an ongoing conservation crisis. Many threatened albatross and petrel species breed and/or forage in Australia's jurisdiction and distribute widely across the world's oceans and remote, offshore islands. These species face a range of threats to their long-term survival on land, particularly habitat degradation and predation by feral species, and at sea, particularly incidental catch during fishing operations. The recovery of threatened albatrosses and petrels relies on an integrated approach involving actions at domestic and international levels. Although Australian breeding and/or foraging populations of albatrosses and petrels, other than the endemic Shy Albatross (Thalassarche cauta), generally represent a small proportion of global populations, these species make a significant contribution to Australia's biodiversity.
The recovery plan recognises that recovery of threatened albatrosses and petrels will take longer than the 10-year life of the current plan due to the long generation lengths and life histories of the species concerned. It further acknowledges that a recovery plan should remain in place for affected species until such time as the conservation status of the species breeding and/or foraging in Australia's jurisdiction has improved to the point where populations are considered secure. The plan aims to provide continuity for recovery actions for these long-lived species, and also recognises that within the five-year timeframe for reviewing progress under a recovery plan there is insufficient time to determine accurately changes affecting the recovery of the species within Australia's jurisdiction. The plan also aims to provide continuity for international advocacy by Australia, particularly through the Agreement on the Conservation of Albatrosses and Petrels, relevant regional fisheries and conservation bodies, and through engaging with range states and international bodies with an interest an expertise in conserving the species.
The long-term vision under this recovery plan is that the populations of albatross and petrel species breeding and/or foraging in Australia's jurisdiction have increased to such a size that the species no longer qualify for listing as threatened under any of the EPBC Act listing criteria.
The objective of the recovery plan is:
To improve the conservation status of albatrosses and petrels so that these species are on a trajectory towards no longer being threatened in Australia's jurisdiction.
The success or otherwise of the recovery plan including the trajectory of species will be measured according to progress on the plan's strategies and overarching actions. The objective will be achieved if within three generations (60 years approx.) there is a measurable and sustained positive population trend (compared to 2021 baseline counts) in the number of mature individuals within the Australian breeding populations of albatross and petrel species within the recovery plan. The recovery plan includes strategies and overarching actions to be applied within Australia's jurisdiction that protect albatross and petrel breeding habitats, address threats to the conservation of the species on land and at sea, generate new knowledge to guide recovery, and increase public awareness. The plan will also assist in the implementation of Australia's international environmental responsibilities, in particular to give effect to obligations under the Agreement on the Conservation of Albatrosses and Petrels and Convention on Biological Diversity, and including engagement with relevant regional conservation and fisheries organisations and arrangements to advance the conservation of albatrosses and petrels.
Strategies have been identified that will be used to measure the effectiveness of the recovery plan and progress towards its objective over the life of the plan.
- Ensure ongoing protection of albatross and petrel breeding sites and habitats in Australia's jurisdiction.
2. Improve the understanding of the size, structure and population trends for albatrosses and petrels breeding in Australia's jurisdiction.
3. Improve effectiveness of management measures that reduce land-based threats to albatrosses and petrels breeding in Australia's jurisdiction.
4. Improve effectiveness of management measures that reduce marine-based threats to albatrosses and petrels foraging in Australia's jurisdiction.
5. Improve understanding of generalised threats to albatrosses and petrels breeding and foraging within Australia's jurisdiction.
6. Improve community awareness of the conservation of albatrosses and petrels.
7. Achieve substantial progress towards global conservation of albatrosses and petrels in international conservation and fishing forums.
This recovery plan is being made jointly by the Australian Government, and the governments of Western Australia, Victoria, Tasmania and Queensland. This joint approach recognises the wide distribution of albatrosses and petrels that breed and forage in Australia's jurisdiction. The approach ensures that complementary actions are taken by the Commonwealth, and by states in areas under their respective control and responsibility. The Australian Government is committed to acting in accordance with the recovery plan and to implementing the plan as it applies to Commonwealth areas.
The recovery plan has been developed with the involvement and cooperation of a broad range of stakeholders, however individual stakeholders have not necessarily committed to undertaking specific actions. The attainment of objectives and the provision of funds may be subject to budgetary and other constraints affecting the parties involved. Proposed actions may be subject to modification over the life of the plan due to changes in knowledge.
The Environment Protection and Biodiversity Conservation Act 1999 (Cth) (EPBC Act) provides for the protection of the environment and conservation of biodiversity, including the protection, conservation and management of listed threatened species through the implementation of recovery plans. Successive recovery plans for threatened albatross and petrel species have been in place since 2001. This National Recovery Plan for threatened albatrosses and petrels (2021) replaces the National Recovery Plan for threatened albatrosses and giant petrels 2011–2016 (DSEWPC 2011b). The plan outlines the research and management actions necessary to stop the decline, and support the recovery of these species so that their chances of long-term survival in nature are maximised.
Seabirds remain among the world's most threatened bird species. Albatrosses and petrels in particular facing an ongoing conservation crisis (Phillips et al. 2016, ACAP 2019a). Many threatened albatross and petrel species breed and/or forage in Australia's jurisdiction and distribute widely across the world's oceans and remote, offshore islands (del Hoyo & Collar 2014, Menkhorst et al. 2017). These species face a range of threats to their long-term conservation status on land: particularly habitat degradation and predation by feral species; and at-sea: particularly incidental catch during fishing operations; as well as generalised threats: particularly marine pollution, marine plastics and climate change (Croxall et al. 2012, Phillips et al. 2016). The recovery of threatened albatrosses and petrels relies on an integrated approach involving actions at domestic and international levels.
This recovery plan provides a national strategy to guide the activities of government, industry, research organisations, and other stakeholders in protecting, conserving and managing threatened albatross and petrel species that breed and/or forage in Australia's jurisdiction. There may be synergies with other work areas or management regimes where opportunities are presented to leverage off existing conservation measures. Where possible collaboration and integration with existing programs should be explored and encouraged.
Although Australian breeding and/or foraging populations of albatrosses and petrels generally represent a small proportion of global populations, other than the endemic Thalassarche cauta (Shy Albatross), these species make a significant contribution to Australia's biodiversity. This plan will be made for each of the following 20 EPBC Act listed threatened species:
Albatrosses and petrels in Australia forage in higher latitude, maritime waters from Western Australia to Queensland, predominantly southwards of the parallel of 25°S, with eight of the species included in this recovery plan breeding on offshore islands of Tasmania (Albatross Island, Macquarie Island, the Mewstone and Pedra Branca), the external Territory of Heard Island and McDonald Islands, and the Australian Antarctic Territory (AAT) (Baker et al. 2002, Menkhorst et al. 2017). Many of these species distribute widely across the world's oceans in the higher latitudes of the southern hemisphere (del Hoyo & Collar 2014). Accordingly, this plan recognises that the recovery of threatened albatrosses and petrels relies on an integrated approach involving actions at domestic and international levels.
The recovery plan also outlines the major benefits to other albatross and petrel species in Australia that will be affected by its implementation. While not listed as threatened under the EPBC Act, these species occur in essentially the same areas, face the same conservation threats, require the same conservation actions, and are listed under the Agreement on the Conservation of Albatrosses and Petrels (ACAP), and included on the Red List of Threatened Species of the International Union for Conservation of Nature (IUCN) (IUCN 2021). Including the following additional albatross and petrel species in this plan makes it a more cogent document:
Phoebastria immutabilis (Laysan Albatross) is not included in this recovery plan. While considered under the previous plan (DSEWPC 2011a, 2011b), available scientific evidence indicates that the species predominantly has a northern Pacific Ocean distribution (ACAP 2012l), and is a rarely detected visitor to Australian waters adjacent to Norfolk Island (Moore 1999). It is not a listed threatened species under the EPBC Act. Consequently, this vagrant species does not warrant inclusion in the plan, and its conservation will not be affected by the plan's implementation.
This recovery plan is being made jointly by the Australian Government, and the governments of Western Australia, Victoria, Tasmania and Queensland. This joint approach recognises the wide distribution of albatrosses and petrels that breed and/or forage in Australia's jurisdiction. The approach ensures that complementary actions are taken by the Commonwealth, and affected states in areas under their respective control and responsibility. The Australian Government is committed to acting in accordance with the recovery plan and to implementing the plan as it applies to Commonwealth areas.
The recovery plan is supported by related frameworks that contribute to the conservation of albatrosses and petrels, in particular:
The recovery plan recognises that recovery of threatened albatrosses and petrels will take longer than the 10-year life of the current plan due to the long generation lengths of the species concerned (Bird et al. 2020). It further acknowledges that a recovery plan should remain in place for affected species until such time as the conservation status of the species breeding and/or foraging in Australia's jurisdiction has improved to the point where populations are considered secure. The plan aims to provide continuity for recovery actions for these long-lived species, recognising that within the five-year timeframe for reviewing progress under the plan there is insufficient time to determine accurately changes affecting the recovery of the species within Australia's jurisdiction. The plan also aims to provide continuity for international advocacy by Australia, particularly through ACAP, relevant regional conservation and fisheries bodies, and through engagement with range states, and international bodies with an interest and expertise in conserving these species.
The Australian Government develops recovery plans for the purposes of the protection, conservation and management of listed threatened species under the EPBC Act. A plan may be made by the Commonwealth or jointly with one or more states and territories in which the threatened species occurs, or with agencies of one or more of those states and territories. Part 13 of the EPBC Act describes the process, content and consultation required when making or varying a recovery plan. A recovery plan may apply to one or more threatened species.
The legislation requires the Commonwealth to implement a plan to the extent to which it applies in areas under Commonwealth control and responsibility. In addition, Commonwealth agencies must not take any action that contravenes a recovery plan. Where a plan applies outside Commonwealth areas in states and territories, the Commonwealth must seek the cooperation of the affected jurisdiction, with a view to jointly implementing the recovery plan.
Successive recovery plans have been implemented to support the recovery of threatened albatrosses and petrels since 2001. Each plan has provided for the research and management actions necessary to stop the decline of, and support the recovery of, the listed threatened albatross and petrel species, so that the chances of long-term survival in nature of the affected species are maximised. Recovery plans made under the EPBC Act are subject to review within five years and sunset after 10 years. The recovery plan for threatened albatrosses and petrels will be the third recovery plan for these species under the EPBC Act, following the Recovery Plan for albatrosses and giant petrels made in 2001 (DEH 2001) and the National Recovery Plan for threatened albatrosses and giant petrels 2011–2016 made in 2011 (DSEWPC 2011a, 2011b).
The taxonomy of albatrosses and petrels has benefited from a significant amount of new taxonomic information that has become available since the initial recovery plan for albatrosses and petrels. The recovery plan uses the taxonomy adopted by ACAP for a variety of reasons including its international standing, use of the most recent data, and review processes. The ACAP taxonomy is the same as that used by the IUCN for the Red List of Threatened Species (IUCN 2021) and the Convention on the Conservation of Migratory Species of Wild Animals (CMS).
Table 1 indicates the relationship between the ACAP taxonomy, and the taxonomy used under the EPBC Act for the albatross and petrel species referred to in the recovery plan. The use of the ACAP taxonomy does not substantially or practically alter the protection, conservation and management actions contained in the plan.
Table 1: Albatross and petrel species referred to in the National Recovery Plan for albatrosses and petrels.
Nomenclature for albatross and petrel species referred to in the recovery plan | Threatened species listing under Environment Protection and Biodiversity Conservation Act 1999 (Cth) |
Species | Common name | Category | Species |
Diomedea amsterdamensis | Amsterdam Albatross | Endangered | Diomedea amsterdamensis |
Diomedea antipodensis | Antipodean Albatross | Vulnerable | Diomedea antipodensis |
Diomedea antipodensis gibsoni |
Diomedea dabbenena | Tristan Albatross | Endangered | Diomedea dabbenena |
Diomedea epomophora | Southern Royal Albatross | Vulnerable | Diomedea epomophora |
Diomedea exulans | Wandering Albatross | Vulnerable | Diomedea exulans |
Diomedea sanfordi | Northern Royal Albatross | Endangered | Diomedea sanfordi |
Macronectes giganteus | Southern Giant Petrel | Endangered | Macronectes giganteus |
Macronectes halli | Northern Giant Petrel | Vulnerable | Macronectes halli |
Phoebetria fusca | Sooty Albatross | Vulnerable | Phoebetria fusca |
Phoebetria palpebrata | Light-mantled Albatross | Not listed | Phoebetria palpebrata |
Procellaria aequinoctialis | White-chinned Petrel | Not listed | Procellaria aequinoctialis |
Procellaria cinerea | Grey Petrel | Not listed | Procellaria cinerea |
Procellaria parkinsoni | Black Petrel | Not listed | Procellaria parkinsoni |
Procellaria westlandica | Westland Petrel | Not listed | Procellaria westlandica |
Thalassarche bulleri | Buller's Albatross | Vulnerable | Thalassarche bulleri |
Thalassarche bulleri platei |
Thalassarche carteri | Indian Yellow-nosed Albatross | Vulnerable | Thalassarche carteri |
Thalassarche cauta | Shy Albatross | Endangered | Thalassarche cauta |
Thalassarche chlororhynchos | Atlantic Yellow-nosed Albatross | Not listed | Thalassarche chlororhynchos |
Thalassarche chrysostoma | Grey-headed Albatross | Endangered | Thalassarche chrysostoma |
Thalassarche eremita | Chatham Albatross | Endangered | Thalassarche eremita |
Thalassarche impavida | Campbell Albatross | Vulnerable | Thalassarche impavida |
Thalassarche melanophris | Black-browed Albatross | Vulnerable | Thalassarche melanophris |
Thalassarche salvini | Salvin's Albatross | Vulnerable | Thalassarche salvini |
Thalassarche steadi | White-capped Albatross | Vulnerable | Thalassarche steadi |
A five-year review of the National Recovery Plan for threatened albatrosses and giant petrels 2011–2016 was conducted with the inclusion of managers, experts and stakeholders (Department of the Environment 2016). The review noted that within the five-year timeframe of the plan there was insufficient time to determine accurately population trajectories of threatened albatrosses and giant petrels. Caution was therefore appropriate when assessing the population status of the 21 albatross and giant petrel species referred to in the plan.
The Review found that of the 28 recovery criteria in the recovery plan, four had been met, while progress had been made against 20 criteria. Recognising that the recovery actions extended well beyond the five-year timeline for the review of the plan, the Review recommended that a future plan should include longer-term objectives and actions. Limited progress against objectives was found to be due to the expected long-term time scale for population recovery and criteria that were infeasible, impractical or unrealistic. The Review noted that of the 12 albatross and petrel breeding populations where monitoring occurred during the life of the plan, one was increasing, two remained stable or increasing, one population was too small to determine its trend, the status of three populations were unknown and five populations were declining. No data had been collected for the remaining eight populations for over 10 years. This was attributed to the logistical difficulties concerning access to those populations. Addressing the lack of data on conservation status for these remaining eight species was identified as a priority action for this recovery plan.
The successful eradication from Macquarie Island by 2014 of pest populations of European rabbits (Oryctolagus cuniculus), ship rats (Rattus rattus) and house mice (Mus musculus) was identified as a major achievement during the review of the recovery plan. This was the largest island eradication project attempted at that time (Hunt G (Minister for the Environment) 2014, Parks and Wildlife Service 2014, Alderman et al. 2019). The Review further noted cats (Felis catus) had been eradicated from Macquarie Island by 2002 (Robinson & Copson 2014).
The Review highlighted that the recovery plan provided a strong policy foundation for action domestically to conduct ongoing monitoring of albatrosses and petrels at key breeding sites in Tasmania (Albatross Island, the Mewstone and Pedra Branca) and at Macquarie Island in the sub-Antarctic.
The Review stressed that in the absence of effective international conservation action, Australia would be unable to secure the conservation of threatened albatrosses and petrels that breed and forage within Australia's jurisdiction. The Review recognised that the recovery plan provided an important framework for international conservation and fisheries forums to establish and improve conservation measures concerning seabird bycatch mitigation in fisheries, and to improve conservation of breeding populations on land.
The Review noted there had been varying degrees of implementation by regional fisheries bodies of conservation measures for seabird bycatch mitigation, and collection of bycatch information through scientific observer programs, and highlighted that further international efforts would be required to improve the effectiveness of these measures over time. Australia's influence and engagement in the work of ACAP was also considered highly influential in addressing threats to albatross and petrel species on land and at sea, including the development of best practice measures and improved conservation advice for range states, as well as states whose vessels fished in the range of albatrosses and petrels.
The Review noted that the threat of marine debris including plastics had increased in significance during the life of the recovery plan, and there is now particular concern about the threat posed by marine plastics to seabirds in the Tasman Sea (Wilcox et al. 2015), an area of high abundance for Australia's albatross and petrel species. The understanding of the potential effects of climate change, particularly concerning the endemic Shy Albatross (Thomson et al. 2015), has also increased during the life of the plan. This threat will likely assume greater significance in future for all species covered by this plan.
The Review concluded that a recovery plan for albatrosses and petrels should remain until such time as populations of affected threatened species breeding and/or foraging in Australia's jurisdiction have improved to the point where the populations are considered secure. The Review considered that the preponderance of key threats to albatross and petrel species remained, with the important exception of feral pest rabbit and rodent species on Macquarie Island that had been eradicated within the life of the existing plan. The potential scope and scale of marine debris including mircoplastics as a conservation threat had increased in light of improved information, however their effects on albatrosses and petrels at the population level would require further research.
The Review considered that an updated recovery plan should include objectives and actions that were ‘SMART’ (specific, measureable, achievable, realistic and timely) and be designed to advance the recovery of threatened albatross and petrel species over the longer term, in line with timeframes for population-level changes to be detected. The Review considered that additional species should be included encompassing those petrel species breeding and foraging in Australian jurisdiction that are listed under the Agreement on the Conservation of Albatrosses and Petrels. The Review concluded that a Recovery Team should be established to discuss implementation and effectiveness of recovery plan actions. The establishment, governance and terms of reference, participation, and frequency of meetings of this forum should be consistent with those for recovery teams for threatened species.
The outcomes of the Review have informed the development of this recovery plan with objectives and actions building upon previous recovery efforts.
Domestic legal frameworks
Commonwealth and state legislation affords protection to threatened albatrosses and petrels in Australia. The species referred to in this recovery plan may be listed as threatened under one or more relevant statutes of the Commonwealth, Western Australia, South Australia, Victoria, Tasmania, New South Wales and Queensland:
The status of each species under Commonwealth and state legislation is set out in Appendix B.
The recovery plan has been developed jointly by the Commonwealth and relevant states under Part 13 of the EPBC Act, and Part 7 of the Environment Protection and Biodiversity Conservation Regulations 2000 (Cth). These set out the content and consultation requirements for making a recovery plan including identifying threats to affected species, and habitats critical to the survival of the of the species.
The importance of albatross and petrel breeding sites in Australia's jurisdiction to the survival of Australia's albatross and petrel species has led to the introduction of access restrictions for all breeding locations in Australia's jurisdiction that are implemented through:
Conservation arrangements
Australia's national efforts under the recovery plan contribute to global work to protect albatrosses and petrels and conserve their biodiversity by range States (Commonwealth of Australia 2019a). The plan assists in the implementation of Australia's international environmental responsibilities, in particular to give effect to Australia's obligations under the Agreement on the Conservation of Albatrosses and Petrels (ACAP) and the Convention on Biological Diversity (CBD). As well, the following additional international and regional conservation arrangements are of relevance to the plan:
Fisheries arrangements
The recovery plan also contributes towards addressing the impacts from high seas fishing activities affecting albatrosses and petrels as associated and dependent species. The following international and regional fisheries arrangements and instruments are of relevance to the plan:
Albatrosses and petrels are mostly surface seizing, pelagic predators that feed on live and moribund prey (Barton 1979, Harper et al. 1985, Harper 1987, Wood 1992, Croxall & Prince 1994). The ability to dive varies across species and involves either surface plunge dives or shallow dives to catch prey (generally <15 m) (Prince et al. 1994a, Hedd et al. 1997, Hedd et al. 2001, Robertson et al. 2006). Some species also prey on other seabirds and scavenge carrion on land (Cherel et al. 2002, Le Bohec et al. 2003).
Albatrosses and petrels are wide-ranging, opportunistic predators. Birds will forage singly and will aggregate in larger numbers where there is a rich food source (Dixon 1933, Brothers 1991). Individual species prefer to feed during the day or at night (often by moonlight) (Hedd et al. 2001, Phalan et al. 2007). Albatrosses and petrels have a diverse diet, depending on the availability of food, including cephalopods, crustaceans, cyclostomes, fish and tunicates, although diet is not well known for several species (Cherel & Klages 1998, Green et al. 1998, Hedd & Gales 2001, Xavier et al 2003a, 2003b). Albatrosses and petrels exhibit a tendency to follow fishing vessels, sometimes in large numbers involving various species (Dixon 1933, Brothers 1991). Competition for fisheries discards and baited hooks can be intense (van der Hoff 2001), with smaller birds subject to secondary attacks by other larger birds (Weimerskirch et al. 1986, Harper 1987, Brothers 1991).
Albatross and petrel species occurring in Australia's jurisdiction predominantly breed on remote, offshore islands in the higher latitudes, with the exception of the Northern Royal Albatross and Westland Petrel that breed on the South Island of New Zealand. The approach to nesting varies between and within species. Some breeding pairs will form solitary nests while others will nest in loose to dense colonies, or in mixed colonies with other seabird species. Nest construction varies between and within species. Nesting strategies include the construction of cupped pedestal mounds, nesting on the ground, slopes and cliffs, and in burrows, as well as nesting among tussock-grass, ferns, grassy meadows, scrub, and under forest canopies.
Albatrosses and petrels are extremely site-faithful (Bried & Jouventin 1999, Gauthier et al. 2010) and the populations currently breeding at identified locations in Australia's jurisdiction are unlikely to breed elsewhere in our jurisdiction (Baker et al 2002). These remote offshore islands therefore should be regarded as habitat that is potentially critical to the survival of albatrosses and petrels in Australia (additional information on each breeding site is provided in Appendix C).
Albatross and petrel species breeding sites in Australia's jurisdiction are as follows:
- Shy Albatross (endemic) breeds on three offshore islands adjacent to Tasmania: Albatross Island, the Mewstone and Pedra Branca (Brothers et al. 2001, ACAP 2013a, 2013d, 2013e, DPIPWE 2021b)
- Black-browed Albatross, Grey-headed Albatross, Grey Petrel, Light-mantled Albatross, Wandering Albatross, Northern Giant Petrel, and Southern Giant Petrel breed on Macquarie Island in the sub-Antarctic (Brothers et al. 2001, ACAP 2013b, DPIPWE 2021a)
- Black-browed Albatross breeds on Bishop and Clerk Islets in the sub-Antarctic adjacent to Macquarie Island (Brothers et al. 2001, Brothers & Ledingham 2008, ACAP 2014)
- Black-browed Albatross, Light-mantled Albatross, and Southern Giant Petrel breed on the External Territory of Heard Island and McDonald Islands in the sub-Antarctic (ACAP 2013c, 2013f). Potentially, a small population of Wandering Albatross might be present on Heard Island (Kirkwood et al. 1989), although only a nest mound was found during a visit in 2001 (ACAP 2013c)
- Southern Giant Petrel breed in the AAT at Giganteus Island (Mac.Robertson Land), Hawker Island (Princess Elizabeth Land), and Frazier Islands (Wilkes Land) (ATCM 2013, 2015, 2016).
The location and extent of albatross and petrel breeding sites in Australia's jurisdiction are provided in Table 2 and under each species profile (see Appendix A). Details of each of the habitats that are regarded as critical to the survival of threatened albatross and petrel species breeding within Australia's jurisdiction are set out in Appendix C.
Table 2: Albatross and petrel breeding site locations in Australia’s jurisdiction.
Site | Species | Location | Coordinates | Approximate size |
Albatross Island | Shy Albatross | 70 km north-west of Stanley, Tasmania | 40°23'S, 144°39'E | 33 ha |
Mewstone | Shy Albatross | 123 km south-west of Hobart, Tasmania | 43°44'S, 146°22'E | 13 ha |
Pedra Branca | Shy Albatross | 111 km south-west of Hobart Tasmania | 43°52'S, 146°58'E | 2.5 ha |
Macquarie Island | Black-browed Albatross, Grey-headed Albatross, Grey Petrel, Light-mantled Albatross, Wandering Albatross, Northern Giant Petrel, Southern Giant Petrel | 1500 km south-east of Hobart, Tasmania | 54°37'S, 158°51'E | 13,000 ha |
Bishop and Clerk Islets | Black-browed Albatross | 33 km south of Macquarie Island | 55°06', 158°41E | 60 ha |
Heard Island | Black-browed Albatross, Light-mantled Albatross, Southern Giant Petrel | 4100 km south-west of Perth, Western Australia | 53°06'S, 73°32'E | 36,800 ha |
McDonald Islands | Black-browed Albatross, Light-mantled Albatross, Southern Giant Petrel | 43 km west of Heard Island | 53°02'S, 73°36'E | 360 ha approx. |
Giganteus Island | Southern Giant Petrel | 16 km west from Mawson station, Mac.Robertson Land, East Antarctica | 67°34'S, 62°29'E | 16 ha |
Hawker Island | Southern Giant Petrel | 7 km south-west of Davis station, Princess Elizabeth Land, East Antarctica | 68°38'S, 77°51'E | 190 ha |
Frazier Islands | Southern Giant Petrel | 16 km north-west of Casey station, Wilkes Land, East Antarctica | 66°13'S, 110°10'E | 60 ha |
Habitat identified as critical to the survival of the Grey-headed Albatross, Shy Albatross, and Wandering Albatross was identified when the initial recovery plan for albatrosses and petrels was developed in 2001 (DEH 2001, 2002, DAWE 2020). Albatross Island, the Mewstone and Pedra Branca comprise the only known breeding habitat for the endemic Shy Albatross. Macquarie Island is the only known suitable breeding habitat under Australia's jurisdiction for the Grey-headed Albatross, and Wandering Albatross. These locations are included in the Register of Critical Habitat under section 207A of the EPBC Act in recognition that the locations are breeding habitat critical to the survival of the species in Australia's jurisdiction, and these populations are important to ensuring the long-term viability and genetic diversity of the species (DEH 2002).
The Key Biodiversity Areas (KBA) program aims to identify, map, monitor and conserve the critical sites for global biodiversity across the planet. This process is guided by a Global Standard for the Identification of Key Biodiversity Areas, the KBA Standard (IUCN 2016). It establishes a consultative, science-based process for the identification of globally important sites for biodiversity worldwide. Sites qualify as KBAs of global importance if they meet one or more of 11 criteria in five categories: threatened biodiversity, geographically restricted biodiversity, ecological integrity, biological processes, and irreplaceability. The KBA criteria have quantitative thresholds and can be applied to species and ecosystems in terrestrial, inland water and marine environments. These thresholds ensure that only those sites with significant populations of a species or extent of an ecosystem are identified as global KBAs. Species or ecosystems that are the basis for identifying a KBA are referred to as Trigger species.
The global KBA partnership supports nations to identify KBAs within their country by working with a range of governmental and non-governmental organisations scientific species experts and conservation planners. Defining KBAs and their management within protected areas or through Other Effective Area-based Conservation Measures (OECMS) will assist the Australian Government to meet its obligations to international treaties, such as the Convention on Biological Diversity. KBAs are also integrated in industry standards such as those applied by the Forest Stewardship Council or the Equator Principles adopted by financial institutions to determine environmental risk in projects.
The initial identification of a site as a KBA is unrelated to its legal status as it is determined primarily based on the distribution of one or more Trigger species at the site. However, existing protected areas will often inform the final KBA delineation, because KBAs are defined with site management in mind (KBA Standards and Appeals Committee 2019). In practice, if an existing protected area roughly matches a KBA, it will generally be used for delineating the KBA. Many KBAs overlap wholly with existing protected area boundaries, including sites designated under international conventions (for example, Ramsar and World Heritage) and areas protected at national and local levels (for example, national parks). However, not all KBAs are protected areas and not all protected areas are KBAs. It is recognised that other management approaches may also be appropriate to safeguard KBAs. The identification of a site as a KBA highlights the site's exceptional status and critical importance on a global scale for the persistence of the biodiversity values for which it has been declared for (particular Trigger species or habitats) and implies that the site should be managed in ways that ensure the persistence of these elements. More information about KBAs is available at: keybiodiversityareas.org/home.
The global KBA partnership currently recognises four Key Biodiversity Areas as important for albatross and petrel conservation in Australia's jurisdiction and to support the long-term persistence of the species. KBAs are also undergoing a regular revision to ensure changes in IUCN Red List status, taxonomic changes, local population trends, as well as increased knowledge of the species are reflected accurately in the KBA network. As such, over time, additional KBAs may be recognised for their importance for albatrosses and petrels or new KBAs may be declared for these and other taxa. Detailed KBA Factsheets, including boundary maps, population estimates of trigger species and scientific references for these seven areas (and other KBAs) are available from the World Database of Key Biodiversity Areas (BirdLife International 2020). The four KBAs in Australia's jurisdiction for albatrosses and petrels include:
Tasmania
- Albatross Island and Black Pyramid Rock — two tiny islands offshore from the north-west of Tasmania. Albatross Island (33 ha) is very rocky, with a coastline of eroded boulders, gulches and caves and a short cover of grasses and herbs across the interior. Black Pyramid Rock (40 ha) is a basaltic rock stack surrounded by steep cliffs, steep grassy slopes and a small central plateau. It is sparsely vegetated as the Australasian Gannet (Morus serrator) has taken most vegetation as nesting material. The endemic Shy Albatross that breeds on Albatross Island is a trigger species for the KBA, with 30-35% of the world population breeding at this location.
- Mewstone — a rocky oval-shaped 9 ha island, about 20 km south of Red Point, between Southport and Port Davey in south-west Tasmania. Composed of Muscovite granite, which is unknown elsewhere in the Southwest National Park, the island is very steep and mostly bare jagged rock. The few plant species present on the island are confined to crevices and cavities in the rock where soil has accumulated. The endemic Shy Albatross that breeds on the Mewstone is a trigger species for the KBA, with more than 60% of the world population breeding at this location.
- Macquarie Island —a sub-Antarctic island located approximately halfway between Antarctica and Australia, about 1450 km south-east of Tasmania and 1300 km north of the Antarctic continent. The listing includes the island and the nearby Judge and Clerk Islets and Bishop and Clerk Islets. The island lies just to the north of an oceanic boundary, the Antarctic Polar Frontal Zone or Antarctic Convergence, where cold polar waters meet warmer sub-Antarctic waters. The island is 34 km long and up to 5 km wide and consists of a long plateau, 200-350 m above sea level, surrounded on all sides by steep slopes or cliffs. The island is free from invasive introduced species, after successive eradication efforts. The islands and seas to three nautical miles are a Nature Reserve, the islands and seas to 12 nautical miles are a World Heritage Area, and 162,000 km2 of seas to the east are in the Macquarie Island Marine Park. The Wandering Albatross, Light-mantled Albatross, Grey-headed Albatross, Black-browed Albatross, Southern Giant Petrel, and Northern Giant Petrel all breed on Macquarie Island and are trigger species for the KBA.
Commonwealth
- Heard Island and McDonald Islands — islands forming an External Territory in the Southern Ocean, over 4000 km south-west of Western Australia and about 1500 km north of Antarctica. Heard Island is 368 km2 and dominated by Big Ben, an active volcano rising to Mawson Peak, at 2745 m. About 70% of the island is covered in snow and glacial ice and black volcanic rocks, with vegetation and bird nests and colonies restricted to the coastal fringe. The McDonald Islands group (~3.6 km2, max altitude 230 m, about 43 km west of Heard Island) includes McDonald Island and other smaller islands and islets. McDonald Island is also volcanic; since 1980, it has doubled in size and grown in height by almost 100 m as a result of lava flows in the 1990s. The islands form part of the 65,000 km2 Heard Island and McDonald Islands Marine Reserve and World Heritage Area that includes the islands, all inshore waters to 12 nautical miles and some offshore waters. The Wandering Albatross, Light-mantled Albatross and Southern Giant Petrel breed on these islands and are trigger species for the KBA.
Albatross and petrel life history strategies are a major factor influencing their conservation. Procellariiform seabirds including the albatross and petrel species in this recovery plan are characterised by their longevity, naturally high adult survival rates, delayed sexual maturity and low fecundity (Warham 1990). Over the 60 years from 1950 to 2010, 324 monitored seabird populations have decreased by nearly 70% worldwide (Paleczny et al. 2015). Changes in adult mortality rates have greater impact on Procellariiform populations than other demographic factors (Croxall & Rothery 1991). Pelagic seabirds are more threatened than coastal resident seabirds, as they tend to have small breeding populations and many are restricted to single islands or island groups (Croxall et al. 2012). When a population cannot be stabilised through local recruitment or immigration, any additional mortality is likely to lead to a decrease of a population (Longcore & Smith 2013). The breeding season of albatrosses and petrels is typically protracted, and if during this time, one parent dies or is killed the death of the dependent offspring may occur, and a loss of breeding opportunities will occur until a new pair bond is established, further jeopardising population viability (Weimerskirch & Jouventin 1987, Croxall et al. 1990). Human activities, such as fishing, disturbance, development and pollution, as well as direct take, either kill seabirds directly or alter the structure and function of their ecosystem (Baum & Worm 2009). Significant modification of their breeding and foraging habitats due to changes to climate have the potential to also affect Australia's albatross and petrel populations directly and indirectly (Tweedie & Bergstrom 2000, Chown et al. 2005, Thost & Allison 2005, Thost & Truffer 2008, Thomson et al. 2015). Albatrosses and petrels are also susceptible to a wide range of diseases. However, the frequency of occurrence and impact of infectious diseases remains largely unknown (Uhart et al. 2018).
Albatrosses and petrels are subject to an array of threats throughout all stages of their life history (Dias et al 2019). These comprise terrestrial threats at breeding sites and marine threats that reduce the survivorship of albatrosses and petrels and/or their capacity to reproduce successfully (Baker et al. 2002, Phillips et al. 2016) (Table 4). Various aspects of albatross and petrel biology and ecology are still not well known. Such understandings are vital to the interpretation and measurement of the likely impact of threats and are important for population viability analysis and other modelling. Despite considerable work, particularly concerning foraging distribution (BirdLife International 2004), details of the breeding biology, feeding ecology, foraging distribution and population trend of many albatross and petrel species are still lacking because of a lack of long-term studies (Phillips et al. 2016). Cumulatively, these terrestrial and marine threats are putting the long-term viability of many species at risk. The threats and issues discussed below are grouped according to subject matter, and do not necessarily appear in order of importance.
The key terrestrial and marine threats to the survival of albatrosses and petrels are set out below. The list is not exhaustive, but identifies the main threats likely to cause one or more of the following adverse impacts affecting the species:
- direct mortality
- indirect loss of reproductive opportunities
- disturbance including effects on nesting and/or foraging behaviour
- morbidity including from injury, disease, and/or contamination
- disorientation through artificial light and ship strikes
- avoidance behaviours
- abandonment of nests
- damage to nesting habitat
- loss of nesting habitat.
The information about the threats to the conservation of albatrosses and petrels is synthesised from a range of sources, noting that this information is likely to change over the life of this recovery plan. Prior to using any listing status against a species, please refer to the threatened species and ecological communities or Species Profile and Threats Database (SPRAT) webpages for current information. These data represent EPBC listed species that have been mapped by the Department and are available for public use, subject to Sensitive Species Policies, as a result of which some maps may be withheld.
Up-to-date information on particular species may be found at the following sources in particular:
NB. The distribution maps show modelled national distributions within the Australian context to support assessment of environmental impacts under the EPBC Act. Species distributions are indicative and not meant for local assessment. Planning or investment decisions at a local scale should seek some form of ground-truthing to confirm the existence of the species or ecological community at locations of interest.
Each of the threats to albatrosses and petrels has been assessed to determine the risk posed to albatross and petrel populations using a risk matrix. This in turn determines the priority for actions outlined below. The threats were considered in the context of the current management regimes. The impact of that threat has been assessed assuming that existing management measures continue to be applied appropriately. If management regimes change, then the level of risk associated with threats may also change.
The risk matrix considers the likelihood of an incident occurring and the consequences of that incident. Threats may act differently in different parts of the species range and at different times of year. Population-wide threats are generally considered to present a higher risk.
The risk matrix uses a qualitative assessment drawing on peer reviewed literature and expert opinion. In some cases, the consequences of activities are unknown. In these cases, the precautionary approach has been applied. Levels of risk and the associated priority for action are defined as follows:
Very high — immediate mitigation action required.
High — mitigation action and an adaptive management plan required.
Moderate — obtain additional information and develop mitigation action if required.
Low — monitor the threat occurrence and reassess threat level if likelihood or consequences change.
Table 3: Risk prioritisation
Likelihood of occurrence | Consequences |
Not significant | Minor | Moderate | Major | Catastrophic |
Almost certain | Low | Moderate | Very High | Very High | Very High |
Likely | Low | Moderate | High | Very High | Very High |
Possible | Low | Moderate | High | Very High | Very High |
Unlikely | Low | Low | Moderate | High | Very High |
Rare or Unknown | Low | Low | Moderate | High | Very High |
Categories for likelihood are defined as follows:
Almost certain — expected to occur every year.
Likely — expected to occur at least once every five years.
Possible — might occur at some time.
Unlikely — such events are known to have occurred on a worldwide basis but only a few times.
Rare or Unknown — may occur only in exceptional circumstances, OR it is currently unknown how often the incident will occur.
Categories for consequences are defined as follows:
Not significant — no long-term effect on individuals or populations.
Minor — individuals are adversely affected but no effect at population level.
Moderate — population recovery stalls or reduces.
Major — population decreases.
Catastrophic — population extinction.
Human disturbance
Threats from human disturbance at or adjacent to breeding sites including direct habitat destruction, damage, and disturbance, as well as interactions with built structures and artificial lighting. |
Coastal development involves the progressive encroachment of natural coastal habitat for human use. Such development has occurred and may occur onshore or on offshore islands. Encroachment of this natural habitat may directly reduce areas used by albatrosses and petrels as breeding sites. Adjacent coastal development may disturb breeding sites due to increased ambient noise, artificial light pollution, and barriers to movement. Additionally, coastal development may directly damage or destroy nesting habitat. As well, disturbance, habitat damage and predation may occur if domestic animals are allowed to stray or enter previously undisturbed natural habitat that is used by albatrosses and petrels as breeding sites.
Australia's albatross and petrel breeding populations are located on remote, offshore islands where coastal development is not presently a significant concern. Prior use of offshore islands, such as for sealing and feather collecting, abated by the early 20th Century. Access to islands where there are breeding populations of albatrosses and petrels is prescribed. Although there are permanent research stations located on Macquarie Island, and near to each of the Frazier Islands, Giganteus Island and Hawker Island in the AAT, all human activities are subject to management plans that aim to avoid or minimise disturbance of albatross and petrel breeding sites.
Coastal development of wind farm turbine infrastructure is an emerging issue, with wind farm proposals being considered for sites along the southern Australian coastline, and on offshore islands, for example in Bass Strait.
Many of the breeding sites for albatross and petrel species that breed beyond Australia's jurisdiction are located on remote offshore islands (Croxall et al. 2012). At some of these locations there are also local human populations with associated direct and indirect impacts on the breeding sites. As well, on the South Island of New Zealand there are breeding populations of Northern Royal Albatross (at Taiaroa Head) (ACAP 2012o) and Westland Petrel (at Punakaiki) (ACAP 2012w), which are also at risk of human disturbance.
Wildlife tourism is a worldwide phenomenon that attracts many visitors, in the early 2000s, more than 700 million usually organised trips were offered worldwide (Higginbottom 2004). However, wildlife tourism takes many forms ranging from non-organised or supervised wildlife observations or interactions to guided and carefully managed ecotourism ventures (Packer & Ballantyne 2013). Among wildlife tourism activities, bird watching ranks very high, and seabird colonies are particularly attractive tourist destinations (for example, Yorio et al. 2001). Wildlife tourism, recreational boating and fishing may disturb albatrosses and petrels at their breeding sites, as well as foraging birds adjacent to these locations.
Introduced invasive species
Threats from non-native species including predation, direct habitat destruction, degradation, damage, or disturbance. |
Albatrosses and petrels are predominantly sensitive to terrestrial habitat changes that affect breeding sites. The progressive spread of human activity across the globe has led to the introduction of non-native animal and plant species to many previously pristine, remote offshore islands including those supporting albatross and petrel populations. While some non-native species were brought to these locations for food, or as domestic pets, other introductions occurred incidentally (for example, rats and mice on board ships). Introduced species may become pest species and threaten seabird populations on affected islands (Jouventin & Weimerskirch 1991, Thiebot et al. 2014).
Albatrosses and petrels are especially vulnerable to introduced mammals because of their lack of effective anti-predator behaviour, their habit of building their nests on the ground or in borrows and leaving chicks unattended during long-range foraging trips, and their low annual productivity (Baker et al. 2002). Invasive fauna may affect seabird populations via nest predation, nest destruction and habitat modification, with introduced grazing and browsing pest species impacting nesting sites by reducing cover, trampling nests and burrows, and increasing the risk of landslips (Croxall et al. 1984, Moors & Atkinson 1984, Robertson & Bell 1984).
Invasive flora species may affect breeding sites of albatrosses and petrels. The invasive flora causes adverse habitat modification, particularly through overgrowing nesting habitat, and creating a barrier to accessing breeding sites, including inhibiting access to burrows.
All breeding sites in Australia's jurisdiction are presently free from introduced species of fauna and flora that may cause population level impacts to albatross and petrel populations. Previous eradication efforts have eliminated feral cats, rabbits, rats and mice from Macquarie Island, the only breeding site in Australia where introduced pest species threatened local populations of albatrosses and petrels, with populations recovering at differing rates (Parks & Wildlife Service 2014, Buller et al in review a, in review b).
While Australian breeding sites are currently free of predators, the risk of introductions is always present, particularly where islands are visited regularly by humans. Ongoing vigilance is needed, in particular, concerning maintaining the human presence at Macquarie Island station, and during occasional visits by tourist vessels to the island, as well as research and management and tourist vessel visits to other islands. Australian and Tasmanian government agencies are working together to achieve the highest level of biosecurity for transport of goods and people to Macquarie Island. The danger to already threatened populations of albatrosses and petrels, in particular, could increase if the introduced pest free status of any breeding site was lost. Quarantine and other regulations are in place at all Australian breeding sites to minimise this threat.
Introduced invasive species remain a significant problem at breeding sites for many of the albatross and petrel species that forage in Australian waters but breed beyond Australia's jurisdiction.
Competition with native species
Threats from competition with native wildlife including direct damage to nesting habitat and predation. |
Albatrosses and petrels, and many other seabirds typically occupy isolated, often relatively small islands. As a consequence, competition (both within and between species) for limited nest space can be intense, particularly on smaller islands.
Interspecific competition for nest space is a potential threat to the Shy Albatrosses breeding population on Pedra Branca where nest sites are limited (DPIPWE 2021b). The number of Australasian Gannets on Pedra Branca has been increasing, while the number of Shy Albatrosses at this location has been decreasing (Bunce et al. 2002, Alderman et al. 2011). Interactions between gannets and albatrosses are still poorly understood, but gannets may be removing nesting material, and displacing Shy Albatross from potential nesting sites, particularly in areas where gannets outnumber albatrosses. Further investigation is underway into the nature and extent of the inter-species interaction, and to explore management options. Following a successful trial on Albatross Island in 2017, 18 artificial nests made of aerated concrete were deployed on Pedra Branca in 2019 at four different sites with cameras set up to monitor each location (DPIPWE 2021b). It is hoped that the artificial nests will improve the breeding success of Shy Albatross nesting on Pedra Branca, as occurred on Albatross Island, where breeding success on artificial nests was found to be double that of natural nests (DPIPWE 2021b).
Disease
Threats from native and non-native pathogens. |
Outbreaks of infectious diseases can have catastrophic consequences for albatross and petrel populations (Barbosa & Palaios 2009). The effect and severity of a disease can depend upon, among other factors, environmental conditions, the strain of the pathogen, the age of a bird, its health status and tends to vary among the various bird species (Woods 2004, Young & van der Werf 2008).
The flea and tick (Ixodes eudyptidis) borne Phlebovirus (Hunter Island Group virus I) was a major cause of Shy Albatross chick mortality during some years at Albatross Island (Woods 2004, Wang et al. 2014, Uhart et al. 2018). Heavily infested nestlings carried ticks clustered around the gape and along the soft, exposed skin on the underside of the bill. Such chicks appeared weak and underweight, and ultimately died (MacDonald & Green 1963, Johnstone et al. 1975). The effects of this avian poxvirus vary inter-annually, and infestations can reduce breeding success to only 10% in some colonies. Thus, disease may be a significant factor restricting the recovery of the Albatross Island population (Johnstone et al. 1975, Woods 2004). Disease links with climate change are likely (Thomson et al. 2015). Ticks on adults and chicks at colonies of Black-browed Albatrosses at the Falkland Islands/Islas Malvinas spread an avian pox virus causing localised sporadic mortality; these ticks are also present on Macquarie Island (Selkirk et al. 1990).
Beyond Australia's jurisdiction, the outbreak of two diseases in the 1980s (Avian Cholera and the pathogenic bacterium, Erysipelas sp.) are notable (Weimerskirch 2004). These caused a decline of the Indian Yellow-nosed Albatross population on Amsterdam Island, a key breeding site for this species, comprising 55% of the global population (ACAP 2012k). These diseases affect mainly young chicks, with a cyclic pattern between years, but also kill adult birds. The diseases potentially also threaten the Amsterdam Albatross and Sooty Albatross breeding populations at this location (Weimerskirch 2004).
The impact of disease on albatrosses and petrels is poorly studied. However, a recent review documented the occurrence of disease vectors in Antarctic seabirds and showed, for example, that 44% of species examined carried gastrointestinal parasites, 33% had potentially harmful bacteria, and 80% were infected with ectoparasites, such as lice (Barbosa & Palacios 2009). DNA analysis of scat samples of Shy Albatrosses revealed a high occurrence of tapeworm and roundworm DNA (McInnes et al. 2017a).
Geological processes
Threats from volcanic activity or earthquakes, including tsunamis and landslips. |
Volcanic activity may impact breeding sites of seabirds through damage or destruction of habitat from eruptions including from resulting lava flows and ash debris. Contrastingly, volcanic activity may result in new environmental niches suitable for colonisation by seabirds.
Active volcanoes on Heard Island and McDonald Island in Australia's sub-Antarctic jurisdiction have erupted several times in recent decades (Stephenson et al. 2005, Commonwealth of Australia 2014). There is evidence of significant displacement of seabirds on McDonald Island following an eruption in 1992 (Crossin et al. 2013). The size of this island has also increased dramatically since then, providing potentially new habitat for seabird species including albatrosses and petrels.
Depending on their magnitude and location, earthquakes, either on land or undersea, may trigger landslips, liquefaction or a tsunami that may lead to damage or destruction of nesting habitat for seabirds particularly nearshore, and ground and burrow nesting petrels. The potential for earthquake effects on Australian breeding populations of albatrosses and petrels is poorly understood. Macquarie Island is seismically active with significant past earthquakes.
The recovery plan recognises that there are no feasible actions that might be taken to mitigate the threat posed by geological processes on albatrosses and petrel populations at affected locations.
Climate variability and change
Threats from climate variability and climate change resulting in significant weather changes beyond historical variance, with effects on life history, breeding behaviour and success, breeding habitat condition, and disease prevalence. |
Climate change is causing major shifts in the distribution of species throughout the world. However, the consequences for ecosystems and community dynamics are not well understood (Albouy et al. 2014). How seabirds will respond to climate change and whether or not they are capable of adapting to new conditions remains largely unknown (Thompson et al. 2015). If species are unable to adapt, they will be vulnerable to extinction, in some cases because climate change will not be the only pressure experienced.
To be able to adapt, species need to have the ability to alter behaviour, and the capacity to evolve and adapt quickly to changing conditions. However, this option is probably limited for albatrosses and petrels, as they are long-lived, exhibit strong fidelity to breeding sites, and the conditions at the breeding sites can change rapidly.
Acquiring information that demonstrates climate change impacts is often not straightforward because of the complexity and interrelationships of environmental variables. For many seabird species long-term studies are not available and base line data are missing. But there are likely to be species-specific responses, because of the variation in life histories and adaptations among seabirds. Shifts in wind patterns, for example, are strongly linked to El Niño/La Niña events in the Pacific Ocean. Incubating Laysan Albatrosses increased their body mass in El Niño conditions and bred more successfully than during La Niña events, while Black-footed Albatrosses (Phoebastria nigripes) did not benefit from these conditions (Thorne et al. 2016).
Climate change is already altering the phenology (timing of life cycle events) of some species. A long-term study (55 years) of a seabird community in East Antarctica found that some species arrived 5-30 days later at their breeding colonies than in the 1950s, while others also laid their eggs a few days later than six decades ago (Barbraud & Weimerskirch 2006). Seabirds have to ensure that resource availability continues to coincide with the critical phases in their breeding cycles (Brown et al. 2016), but the phenology of their prey does not necessarily alter to the same extent. This could lead to a mismatch in timing of breeding and food availability. Alternatively, seabirds may shift their distribution if conditions have become more favourable elsewhere. However, such biogeographic shifts, if possible, result in major changes to ecosystem structure, species abundance and biodiversity (Beaugrand 2014).
Different effects of climate change are already observed among birds of the same species but of different ages. For example, among Black-browed Albatross monitored for over 40 years, the survival of middle-aged birds was reduced in years when sea surface temperatures in foraging areas were elevated, while old and young albatrosses survived better than in cold years (Pardo et al. 2013). As well, while generally regarded as monogamous, pair separations of Black-browed Albatross have increased at New Island in the Falkland Islands/Islas Malvinas from 1% to 8% across years due to warmer seas surface temperature anomalies arising from climate change environmental variability (Ventura et al. 2021).
The breeding success of Amsterdam Albatrosses, monitored annually from 1983 to 2006, was reduced in years of elevated sea surface temperatures in spring and summer, while warmer sea surface temperatures in the species’ wintering grounds did not appear to have a measurable effect (Barbraud et al. 2011). Further understanding of how populations will respond to climate change will be necessary for a successful assessment of impacts on breeding performance, success and survival.
Climate change may also act as a catalyser of epizootics, especially infectious diseases (Altizer et al. 2013). The effect of changing patterns of transmission of infectious diseases, such as Avian Cholera (Uhart et al. 2018), may pose a major threat for albatrosses and petrels in the future, especially in the Southern Ocean environment where ecosystems have evolved in isolation (Weimerskirch 2004, Phillips et al. 2016).
Climate change may have deleterious weather-based effects including elevated temperatures (heatwaves), changes in rainfall patterns, and storm surges (Thompson et al. 2015, Phillips et al. 2016). These may adversely affect nesting habitat, through the loss of moisture for plants used in building nests. At Heard Island, climate change is having a dramatic impact including changes in weather patterns and glacial retreat, with vegetation and lagoons now existing where once there were sea-front, glacier snouts (Thost & Allison 2005, Thost & Truffer 2008).
Local climatic conditions have been shown to impact breeding success and the population status of the endemic Shy Albatross, and impacts are likely to increase in a changing climate (Thomson et al. 2015). A current doctorate research project aims to further the ecological understanding of Shy Albatross and the influence of climate variability and change to inform a decision-making framework to guide future conservation and management of the species (DPIPWE 2021b).
Storms and cyclones can seriously affect the nesting substrate, vegetation and wildlife on islands, in addition to affecting seabirds at sea. Such natural factors place additional pressures on seabird populations. At Pedra Branca significant storm surges can occur that overwash this Shy Albatross breeding site (DPIPWE 2021b). On the Sisters, the Pyramid and Forty-fours Islands in the Chatham Islands Group (New Zealand), a severe easterly storm in 1985 stripped the islands bare of vegetation and soil cover (Robertson 1998). Chatham Albatrosses breeding on these islands were unable to construct proper nest sites, and consequently egg mortality increased significantly. To compound the problem, most of the breeding population of the normally biennially nesting Northern Royal Albatross now nests annually owing to low breeding success, thus further limiting nest site availability (Robertson 1998, Taylor 2000).
The potential for degradation and loss of suitable habitat due to climate change is a threat to all Australian albatrosses and petrels. Management of this threat requires both domestic and international action. Long-term monitoring studies are needed to assess status and trends of albatross and petrel populations, as well as changes in their phenology and distribution. Monitoring of environmental conditions in parallel with albatross and giant petrel breeding parameters will help to determine any correlation. The potential for intervention options has been examined for the endemic Shy Albatross (Alderman & Hobday 2017). Interventions including artificial nests and use of avian-friendly pesticides have been shown to increase breeding success and are potential responses to assist the species to adapt to climate change (DPIPWE 2021b).
Fisheries interactions and bycatch
Threats from interactions between seabirds and fishing gear. |
Seabirds are opportunistic predators and scavengers of surface and near surface prey (Harper et al. 1985), and follow fishing vessels to scavenge on discards and baited hooks, and prey on fisheries catch during hauling (Baker et al. 2002, Patrick et al. 2015, Collet et al. 2017, Collet & Weimerskirch 2020). Seabirds interact with fishing gear either by being struck by, hooked on, or entangled in the fishing gear (DAWR 2018), which may result in direct mortality or serious injuries leading to subsequent death (Huin & Croxall 1996, Commonwealth of Australia 2018b). Direct mortalities from interactions with fishing gear may lead to a loss of reproductive opportunities from the death of offspring if the remaining adult is unable to adequately incubate the egg, or provision the chick before fledging, and from lost breeding opportunities while the remaining adult seeks to establish a new pair bond to recommence breeding.
Incidental mortality from interactions with fishing operations is a widespread, pervasive, high-impact threat to pelagic seabirds, particularly albatrosses and petrels (Croxall et al. 2012, Phillips et al. 2016). High rates of seabird bycatch occur in a wide range of longline (Brothers 1991, Gales 1998, Anderson et al. 2011), trawl (Bull 2007, Croxall et al. 2012), purse seine (Baker & Hamilton 2016, Suazo et al. 2017), and gillnet fisheries (Žydelis et al. 2013).
Available evidence highlights the threat posed to seabirds from fisheries. Anderson et al. (2011) estimated at least 160,000, and potentially 320,000, seabirds are killed by longlines annually. In 2016, an estimated 36,000 seabirds (and potentially up to 110,000 seabirds) were bycaught in pelagic longline fisheries in waters southwards of the parallel of 25°S (BirdLife International (South Africa) 2019). Global estimates for seabird mortalities in trawl and purse seine fisheries are not available. Žydelis et al. (2013) estimated at least 400,000 seabirds die in gillnets each year.
Seabird bycatch levels are likely to be significantly underestimated due to cryptic mortalities. Interactions with pelagic longlines may have killed twice the number of seabirds than previously thought due to cryptic mortalities (Brothers et al. 2010, Debski & Pierre 2014). At least 23% of total mortalities were not observed during demersal trawl fishing (Parker et al. 2013).
Conservation and research efforts have been, and continue to be, directed towards understanding interactions between seabirds and fishing gear and preventing population declines from this threat (Bull 2007, Croxall et al. 2012). Effective mitigation measures have been developed for longline fisheries and are undergoing further refinement and development of cost-effective alternatives (FAO 1999, Commonwealth of Australia 2018b, ACAP 2021a, 2021c). Progress is also being made into effective mitigation methods for trawl, purse seine and gillnet fisheries, but this is less advanced than longline fisheries (FAO 2009, Maree et al. 2014, Parker & Molloy 2017, Suazo et al. 2017, ACAP 2019c). Challenges remain in encouraging various international fisheries management bodies to adopt best and improving mitigation technologies and techniques and verify compliance (Phillips et al. 2016, ACAP 2019a).
Longline fishing operations
Longline fishing involves the setting and hauling of one or more lines (mainline) that contain many individual hooks on branch lines (or snoods). The mainline can either be anchored or drifting, oriented vertically or horizontally, and varies considerably in length and number of hooks. Longline fishing can be pelagic (set midwater) or demersal (set on the seabed).
Longline fishing still is a major threat affecting albatrosses and petrels because of the high level of incidental mortality (Yeh et al. 2013). Each year, many thousands of albatrosses and petrels are accidentally killed on hooks deployed by pelagic longline fishing vessels (Baker et al. 2002). In demersal longline operations, where hooks are deployed at a much faster speed and are attached to shorter snoods than in the pelagic operations, seabirds get caught by the fast-descending hooks or entangled in branchlines when chasing after bait (Robertson et al. 2006). Although most mortality occurs directly when birds are caught during line-setting and, less commonly, hauling, albatrosses and petrels may also die after they are released with critical injuries (Huin & Croxall 1996), or following ingestion of fishing hooks when birds swallow discarded baits and fish heads containing hooks. In most cases, the death of breeding adults will lead to the subsequent death of dependent chicks.
All species of albatross and petrel breeding in Australia's jurisdiction may be bycaught during longline fishing operations (Thiers et al. 2014, Thiebot et al. 2015, Phillips et al. 2016). The implementation of successive Threat Abatement Plans for the incidental catch (or bycatch) of seabirds during oceanic longline fishing operations have significantly reduced seabird bycatch in Commonwealth-managed fisheries (Commonwealth of Australia 2018b).
The Threat Abatement Plan establishes performance criteria for each Commonwealth-managed longline fishery, and describes the management actions that the Australian Fisheries Management Authority will take if these criteria are exceeded. The plan highlights that to continue the downward trend and minimise or avoid bycatch, further innovation in mitigation will be necessary (Commonwealth of Australia 2018b). For example, in the Eastern Tuna and Billfish Fishery trials are underway of several new and/or modified seabird mitigation measures to further reduce bycatch rates (FRDC 2020).
For Australian longline fisheries in the sub-Antarctic at Macquarie Island, and Heard Island and McDonald Islands, additional management measures are implemented by the Australian Fisheries Management Authority to protect local breeding populations of seabirds. These measures include, but are not limited to, seasonal closures during the peak breeding season (for species breeding during the Austral summer), seabird bycatch limits, internally weighted longlines, retention of offal, and on-board observers of fishing operations. The additional measures have led to very few seabirds now being bycaught in the two fisheries.
Contrastingly, a significant threat remains in areas beyond Australia's jurisdiction (Phillips et al. 2016, Abraham et al. 2019). As well, the distribution of some albatrosses and petrels breeding in Australia's jurisdiction overlaps extensively with longline fisheries in areas beyond Australia's jurisdiction (Birdlife International 2004). Compliance with mitigation measures remains a key issue as non-compliance leads to significant increases in seabird bycatch.
A key mechanism for promoting and ensuring compliance in high seas fisheries is through independent monitoring of fishing operations. However, for many of the world's fisheries, observer coverage is either non-existent or falls below levels required to estimate seabird bycatch levels accurately (Dietrich et al. 2004, Small 2005, Baker et al. 2007). The use of electronic monitoring systems that employ cameras to independently monitor fishing operations, show potential in improving reporting of seabird bycatch by vessel operators (Tremblay-Boyer & Abraham 2020). However, the utility of electronic monitoring in high seas fisheries is unclear, as fishing vessels may be absent from home ports for extended periods, and the retrieval and audits of footage may be considerably delayed.
According to circumstance, a range of mitigation measures have been developed to reduce the incidental catch of seabirds in longline fisheries (Brothers et al. 1999, Dietrich et al. 2004, Bull 2007). They include night setting, line weighting, bird scaring lines, seasonal and/or area closures, avoidance or control of offal discharges, and hook shielding devices (Commonwealth of Australia 2018b, ACAP 2021a, 2021c). The mitigation measures reduce bycatch mainly during setting, by increasing the sink rate of baited hooks to get them quickly out of the reach of diving seabirds, and by minimising the congregation of seabirds around vessels. Innovation in seabird bycatch mitigation in longline fisheries is ongoing (for example, Robertson et al. 2014).
Each measure has different attributes, costs and potential to reduce seabird catch successfully. However, in most longline fisheries, the greatest reduction in bycatch comes from using a combination of measures. Some measures, such as night setting and line weighting, have been consistently successful in a number of longline fisheries, while the effectiveness of others has varied between vessels and seabird species (ACAP 2021a, 2012c).
Trawl fishing operations
Seabird mortality may be higher in trawl fisheries than in longline operations, at least in some areas of the globe, due to the higher fishing effort in this sector (Favero & Seco Pon 2014). Seabird mortality arises where birds are struck by warp wires (Sullivan & Reid 2002, 2003) or net sonde cables (Weimerskirch et al. 2000, Wienecke & Robertson 2002), or become entangled by these or the trawl net (Sullivan et al. 2006b). The seabird may be killed by the collision, or drown, or die later from its injuries (cryptic mortality). The type of trawl fishing and the time of year are important factors influencing the extent of seabird mortality. The problem of interactions with trawl gear is exacerbated when large numbers of birds are present around vessels, such as may occur when offal is discharged during processing of catch. Only a small proportion of the birds killed are hauled on board during fishing operations (Abraham 2010, Debski & Pierre 2014, Parker et al. 2013).
A major limitation in the gathering of accurate data is the lack of independent observer programs in trawl fisheries that would aid in the determination of lethal and sub-lethal seabird interactions with trawl gear. Reliable data on the levels of seabird bycatch in Australian trawl fisheries would require observer programs and/or greater levels of electronic monitoring to be established to specifically focus on this issue.
Although in Australia's trawl fisheries seabird bycatch appears to be relatively low, large numbers of albatrosses and petrels are killed in trawl fisheries worldwide (Sullivan & Reid 2002, González et al. 2006, Sullivan et al. 2006b, Baker et al. 2007, Bull 2007, Croxall et al. 2012, Paz et al. 2018). The foraging distribution of some albatrosses and petrels breeding in Australia's jurisdiction overlaps extensively with trawl fisheries in areas beyond Australia's jurisdiction (Birdlife International 2004). At times, seabirds attend fishing vessels in distant fisheries in large numbers where they are exposed to collision and entanglement risks (Wienecke & Robertson 2002, González et al. 2006, Baker et al. 2007).
At Macquarie Island, a single trawler operated in waters adjacent to the island from 1994 to 2009. There have been no deaths of albatrosses and petrels recorded. During trawl fishing at Heard Island and McDonald Islands only a few seabird deaths were recorded (Lawton et al. 2007). There is considerable trawl fishing elsewhere within Australian waters with vessels targeting a range of deep-water crustacean and finfish species. Much of this fishing effort occurs within areas frequented by albatrosses and petrels, including areas near important breeding colonies around Tasmania (Sagar et al. 2000). Because of high levels of cryptic seabird mortality in trawl fisheries, independent observers have been specifically tasked with quantifying seabird interactions (Baker et al. 2002, Sullivan et al. 2006a).
Since these investigations identified significant levels of albatross and petrel bycatch, the Australian Fisheries Management Authority now requires all vessels to develop an appropriate seabird management plan to manage risk factors, such as offal discharges, and to employ appropriate mitigation measures, to help reduce bird interactions with trawl warp wires (AFMA 2022b).
Studies to determine the effectiveness of seabird mitigation measures in trawl fisheries are not as advanced as in longline fisheries (FAO 2009, ACAP 2021b). Not discharging offal and limiting factory discharge to 'dirty water’ when the trawl gear is in the water reduces interactions between seabirds and gear as less birds are attracted to the vessel (Sullivan et al 2006a ACAP 2021b). Various devices have been developed to reduce warp and net sonde cable strikes (for example, Løkkeborg 2011). Bird scaring lines can also be effective (Sullivan et al. 2006a, Melvin et al. 2011). A combination of mitigation measures is recommended to reduce seabird mortality (Bull 2007, ACAP 2021b). Innovation in seabird bycatch mitigation in trawl fisheries is ongoing (for example, Koopman et al. 2018), however, further studies are required to identify and determine the efficacy of new mitigation technologies and techniques.
Gillnet fishing operations
Globally, gillnet or driftnet fisheries have a significantly higher bycatch rate for seabirds, marine mammals and sea turtles than longline and trawl fisheries (Lewison et al. 2014). Due to the excessive levels of bycatch (approximately 500,000 seabirds per year in the North Pacific alone) (Eisenbud 1985), the UN General Assembly called for a global moratorium on pelagic driftnetting in 1991 (Resolution 44/225), with many nations, including Australia, implementing legislation to ban large-scale driftnetting. Some illegal setting of driftnets still occurs, mainly in the northern hemisphere (Žydelis et al. 2013). Small-scale gillnet fisheries still operate in many EEZs and harm bird populations. In the Baltic and North seas alone, they may kill up to 200,000 waterbirds per year (Žydelis et al. 2009).
Demersal gillnets that touch the sea floor are the only type of gillnet permitted in Commonwealth fisheries (AFMA 2022a). These nets are usually set in waters less than 100 metres deep, and are used by commercial operators and recreational fishers. Although most states in Australia have declared the use of recreational gillnetting illegal, it is still permitted in Western Australia and Tasmania (Lyle et al. 2014). In Australia, the impact of gillnetting on albatrosses and petrels is only recently emerging in Commonwealth-managed fisheries where electronic monitoring records are available (see AFMA Protected Species Interaction Reports). The impact of recreational gillnetting on albatrosses and petrels is unlikely to be significant as nets are set in nearshore waters where these birds do not usually occur.
The significance of albatross and petrel bycatch by global smaller driftnet and fixed gillnet fisheries is unknown, and of concern. Few seabird bycatch reduction methods have been developed for gillnet fisheries, although increasing the visibility of the net reduces seabird bycatch for some species (Bull 2007). Further studies are required to identify and determine the efficacy of mitigation technologies and techniques, and potential management options to reduce the impacts of gillnet bycatch.
Deliberate take
Threats from intentional killing of adults and juveniles. |
Although it is an offence under the EPBC Act and related state legislation, threatened albatrosses and petrels are sometimes intentionally shot for sport by recreational fishers or to reduce scavenging from commercial fishing vessels both inside and outside of Australian waters (Blakers et al. 1984, Tomkins 1985, Adams 1992). The prevalence of such practices and the extent to which they persist is unknown.
Beyond Australia's jurisdiction, there are reports of incidental and intentional take of albatrosses for purposes of sport, food, and use as bait (Phillips et al. 2016), in artisanal and commercial longline, gillnet, and jigging fisheries in waters off South America (Stagi et al. 1998, Awkerman et al. 2006, Alfaro-Shigueto et al. 2016), and southern Africa (Adams 1992).
There are also reports of bill mutilation of albatrosses and petrels in the southwest Atlantic Ocean associated with the removal of hooks from bycaught birds (Gianuca et al. 2020). These mutilation practices cause morbidity and eventual death of affected birds, and are occurring despite the widespread availability of guidelines for the safe removal of hooks from bycaught seabirds (ACAP 2019b).
Marine pollution
Threats from marine pollution, contamination and debris, including plastics and microplastics. |
Fuel and oil spills
Bulk fuel and/or oil spills are caused through a variety of factors, such as groundings, explosions, collisions or the blowing out of wellheads near drilling sites. Oil spills can have catastrophic impacts on seabird populations. One of the most widely publicised spills was caused by the grounding of the Exxon Valdez in Prince William Sound, Alaska, in 1989 where 37,000 tonnes of crude oil was lost into the marine environment (Piatt & Ford 1996). Over the six months following the spill about 30,000 carcasses of oiled birds were collected. However, many affected birds are never found as they die at sea, and those collected present only a small percentage of the actual mortality. Some 250,000 birds are estimated to have perished as a consequence of the Exxon Valdez oil spill (Piatt & Ford 1996). These effects continue well after the spill (Irons et al. 2000), with many affected seabird populations showing no sign of recovery and some species continuing to decline a decade later (Lance et al. 2001).
The extent of the environmental damage depends upon the location and the time of a spill. For example, in July 1995, the Iron Baron struck a reef near Low Head in northern Tasmania. About 550 tonnes of bunker oil were spilled over 20 days. This event occurred in Tasmania's most important breeding area of Little Penguins (Eudyptula minor) whose breeding season had just started. More than 2100 birds were rescued, the majority of which were penguins (Goldsworthy et al. 2000).
Diving seabirds and surface feeders are negatively affected by oil spills. When seabirds dive through or feed on an oil slick the body becomes physically smothered compromising the bird's insulation and waterproofing which increases heat loss, often resulting in mortality. Severely oiled birds are unable to hunt and capture prey efficiently and the matted plumage reduces the bird's buoyancy and may cause them to sink and drown (Baker 1983, GESAMP 1993).
Furthermore, petroleum products are toxic to birds when ingested. Seabirds swallow oil either when they preen the soiled feathers or when feeding, for example, through scavenging oiled carcasses. Bunker and crude oils irritate intestines affecting the uptake and transport of electrolytes and water. This can retard the growth of chicks (Boersma et al. 1988). Intestinal irritations can also lead to bleeding (Fry & Lowenstine 1985). Adrenal and other glands are also negatively affected by oil ingestion as are liver and kidneys. Ingested toxins suppress the immune system of birds and reduce their reproductive efforts (Burger & Fry 1993, Burger & Gochfeld 2002).
On a broader ecological scale, oil may be retained in sediments for many years, leading to the temporary or permanent loss of species critical to the ecological balance of an ecosystem. In addition, crude oil is essentially a mixture of many hydrocarbon compounds, some of which are toxic and/or persistent. These can accumulate in the marine food chain (described above) and may potentially lead to lethal or sub-lethal changes in metabolic functions (Baker 1983, GESAMP 1993). Albatrosses and petrels are highly vulnerable to the hazards of oil or fuel spills as they spend much of their foraging time on the sea surface (Baker et al. 2002).
Chemical contaminants
The two broad categories of chemical pollutants are persistent organic pollutants (POPs), such as polychlorinated biphenyls (PCBs) and polychlorinated naphthalenes (PCNs), and heavy metals, such as mercury and cadmium. PCBs and PCNs are a mixture of chlorinated compounds that do not occur naturally. These compounds are highly toxic and, depending on concentration and exposure time can lead to a variety of serious health effects (Agency for Toxic Substances and Disease Registry 2000).
POPs and heavy metals are now global contaminants reaching regions far from their original areas of production or use. Elevated levels of POPs and heavy metals have been found in the plasma of adults, chicks and eggs of seabirds from every continent (including Antarctica) and virtually all islands across the globe (Croxall et al. 1984, Auman et al. 1997, Ludwig et al. 1998). Carrion feeders, such as albatrosses and petrels, had noticeably higher levels (Becker et al. 2016). POPs and mercury contamination can lead to reduced hatching and fledging success, and can disrupt the endocrine system, that is the secretion of hormones that regulate behaviours (Tartu et al. 2015, Goutte et al. 2014). As albatrosses and petrels are long-lived and typically highly dispersive, they tend to accumulate high levels of chemical contaminants (Muirhead & Furness 1988, Luke et al. 1989, Lock et al. 1992, Auman et al. 1997, Ludwig et al. 1998, Stewart et al. 1999), with the potential risk of population declines (Luke et al. 1989, Auman et al. 1997, Ludwig et al. 1998).
A number of albatross and petrel species carry unusually high concentrations of heavy metals, in particular cadmium and mercury (Muirhead & Furness 1988, Luke et al. 1989, Lock et al. 1992, Thompson et al. 1993, Stewart et al. 1999, Hindell et al. 1999, Becker et al. 2002). Mercury levels in the liver in Wandering, Sooty and Northern and Southern Royal albatrosses are among the highest recorded for free-living birds (Stewart et al. 1999, Goutte et al. 2014).
Marine debris
Marine debris comprises a variety of materials including timber, glass, rubber, metal and plastics. An estimated 80% of marine debris originates from terrestrial sources, and a large percentage of it is plastics (Allsop et al. 2006). Marine debris from human sources is increasing in scale and distribution across ocean systems globally (Andrady 2011, Jambeck et al. 2015, Roman et al. 2020). Low density plastic particles will float on the sea surface. For example, in the early 2010s, the concentration of plastic particles on the ocean surface around Australia averaged about 4260 pieces per square kilometre, but ranged from zero to around 50 000 pieces per square kilometre (Reisser et al. 2013), and is predicted to rise significantly in coming decades (Wilcox et al. 2015). Marine plastic pollution is now ubiquitous as wind and currents carry the debris into remote regions. Over time, plastics break up into smaller and smaller particles while the total quantities are still increasing (Barnes et al. 2009).
Marine plastic pollution impacts seabirds in a variety of ways. However, the rate of this source of morbidity and mortality remains unknown (Laist 1997, Kühn et al. 2015). Entanglement in discarded or lost fishing lines and abandoned nets, leads to drowning or the inability to escape predators, and ingestion of plastics can have lethal or sub-lethal effects (Coe & Rogers 1997). Sharp particles can damage the gut lining or irritate stomach tissue when ingested; resulting infections often lead to death (Kärrman et al. 2016). The presence of plastic debris in the gastrointestinal tract may reduce feeding due to perceived satiation and lead to a loss of condition and starvation (Pierce et al. 2004, Senko et al. 2020). The debris can also cause mechanical blockage or impairment of the digestive system, resulting in starvation. Some plastics are also a source of toxic pollutants, which are released into the bloodstream as the bird's digestive system attempts to break down the substance (Ryan et al.1988). The subsequent reduction in fitness can lower the bird's ability to reproduce successfully, catch prey and/or avoid predation (Fry et al. 1987, Sileo et al. 1990).
Plastics adrift in the ocean are covered in biofilm, a thin layer of microorganisms that expel dimethyl sulphide (DMS). This compound attracts albatrosses and petrels as the prey items of these birds exude the same olfactory material (Savoca et al. 2016). In consequence, seabirds are highly likely to mistake plastic particles for food and ingest them. Albatross and petrel chicks receive pollutants when they receive regurgitated food. Due to the physical impaction and internal ulceration, the chicks receive less food and nutrients which increases their chances of starvation and dehydration (Fry et al. 1987, Sileo et al. 1990).
Plastic ingestion affects some Australian albatross and petrel species (Baker et al. 2002, Gall & Thompson 2015, Roman et al. 2020). For example, analyses of 540 Shy Albatross chicks that had died 1% had plastic debris in their stomachs, ranging from segments of plastic bags to solid, coloured pieces of plastic (Hedd & Gales 2001). Black-browed, Grey-headed, Wandering Albatrosses and Southern Giant Petrels have all been observed regurgitating plastic debris to their chicks at breeding sites outside of Australia (Huin & Croxall 1996). In the southern hemisphere, plastic ingestion also affects Antipodean, Atlantic Yellow-nosed, Black-browed, Buller's, Chatham Island, Indian Yellow-nosed, Light-mantled, Northern Royal, Salvin's, Sooty, Southern Royal, Wandering and White-capped albatrosses, and Black and White-chinned petrels (Ryan 1987, Robertson 1998, Gall & Thompson 2015, Roman et al. 2020). In the waters off Australia and New Zealand, marine plastics ingestion is estimated to increase mortality in some albatross species by up to 3.4%, a level expected to lead to population declines, particularly in those populations already affected by other threats (Roman et al. 2020). It is likely that most or all other albatross and petrel species ingest plastic debris.
Microplastics can enter the food chain and can lead to secondary ingestion. For example, small plastic particles taken up by zooplankton can bioaccumulate and concentrate in higher vertebrates (Cole et al. 2013). The feeding ability of zooplankton is hampered when the guts are full of debris, and their health and function are compromised (Cole et al. 2013). It is currently not known whether toxins associated with plastics are passed through the food chain, and whether there are any potential negative effects of this transport.
Competition with fisheries for prey species
Threats from competition with fisheries for prey species. |
Global captures of marine living resources are reasonably well documented. For example, in 2013, marine fisheries reported the catch of 80.9 million tonnes of fish globally (FAO 2016). Stocks fished biologically sustainably have decreased from 90% in 1974 to 69% in 2013, 58% of stocks are deemed to be fully fished, that is any increase in effort would make these fisheries no longer sustainable (FAO 2016). As the percentage of fully fished stocks has increased, the proportion of under-fished stocks has decreased (FAO 2016). Global catches vary with region—among the most productive areas is the northwest Pacific Ocean where 21.4 million tonnes (27% of global marine catch) were landed—while in comparison, in the southeast Pacific Ocean only 8.9 million tonnes (11%) were caught (FAO 2016). Illegal, unreported and unregulated (IUU) fishing activities add significantly to these numbers. For example, IUU catches are probably highest off western Africa and are responsible for 40% of total regional catches (Agnew et al. 2009). Thus, the impact on marine ecosystems is substantial and can lead to a shift in abundance of species not targeted by commercial fishers.
There is extensive overlap between areas exploited by commercial operations and seabird foraging areas (BirdLife International 2004). However, competition occurs only where commercial fishers and seabirds are targeting the same species in the same area (noting that interactions also occur between seabirds and fishing gear to scavenge catch during hauling). Intensive industrial fishing puts acute pressure on European seabirds that rely on the West African fish resources in winter (Gremillet et al. 2015).
In Australia, primary prey species for Shy Albatrosses include Redbait (Emmelichthys nitidus), Gould’s squid (Nototodarus gouldi), and Jack mackerel (Trachurus declivis) are all commercially harvested (Hedd & Gales 2001). The Fisheries Status Report relevant to these commercial species indicates they are currently 'not overfished’ in Australian waters (ABARES 2021). The ecological sustainability of fisheries should include consideration of dependent seabird species, particularly as commercial fishers target species at increasingly lower trophic levels (Pauly et al. 1998).
Many commercial fisheries target large predatory fish, such as tunas and billfishes, (Juan-Jordá et al. 2011). The removal of large numbers of predators is likely to alter the structure of marine food webs, but does not necessarily lead to a decrease in the stocks of prey fish species (Danckwerts et al. 2014). The impact on seabirds is more indirect. Large seabirds, such as albatrosses and petrels, are unable to dive to more than a few metres, but many prey species occur at depths of around 50 m. To access these fish species, many seabird species rely on subsurface predators to drive fish schools to the surface where they then become accessible to foraging seabirds (for example, Le Corre & Jaquemet 2005). Thus, as levels of commercial harvesting of large marine predatory fish species increase, seabirds are less likely to access food resources.
No albatross or petrel species within the recovery plan is presently identified as being at risk from competition with fisheries for food resources. It is difficult to determine the level (and the effects) of competition for food resources between seabird populations and fisheries (Hedd & Gales 2001) mainly because baseline data are often limited or unavailable. However, taking into account the needs of associated and dependent species is consistent with the principles of ecologically sustainable development. Exercising a precautionary approach with regard to the impact of fishing activities is needed to ensure the long-term sustainability of the marine environment.
Dependence on fisheries discards
Threats from reliance on fisheries discards. |
Discards from fishing operations can alter ecosystem functions (Fondo et al. 2015). Seabirds opportunistically feed on easily available food items either discarded during commercial fishing operations or offered by tourist operators to enable tourists a better viewing opportunity. These resources, however, can affect community structures, population dynamics and the fitness of individuals (Oro et al. 2013), and may alter the manner in which seabirds use the seascape (Bartumeus et al. 2010). This is particularly the case for scavenging seabirds.
Some seabird species have become dependent upon the offal discarded from fishing vessels during operations and/or processing at sea. They scavenge dead prey and fishery discards and bait (Croxall & Prince 1994). The disposal of offal encourages albatrosses and petrels to follow fishing vessels, significantly increasing their likelihood of becoming injured or killed during fishing operations by direct interactions with fishing gear. As well, some populations have become habituated to the regular food source and appear to have altered their foraging ranges and dynamics accordingly (Ryan & Moloney 1988, Adams 1992, Blaber et al. 1998, Weimerskirch 1998, Sagar et al. 1999).
Discards are a key food resource for many seabird species (Votier et al. 2004). Black-browed Albatross foraging over the Patagonian Shelf during the non-breeding season appear to exploit fishery discards, particularly of trawlers (Mariano-Jelicich et al. 2014). The additional food obtained from commercial fishing operations may positively influence breeding success and, hence, population sizes in some seabird species (for example, Blaber et al. 1998).
Bans on discards have been introduced in various fisheries worldwide to eliminate the waste of marine resources and encourage more selective fishing (for example, Condie et al. 2014). However, cessation of discarding may have negative effects on seabird populations, at least in the short-term (Baker et al. 2002). For example, in the North Sea, discard bans, particularly when coupled with reduced availability of small shoaling pelagic fish, can result in an increase in predation by Great Skua (Catharacta skua) on other birds (Votier et al. 2004). Accordingly changes to discarding practices should be introduced gradually rather than abruptly, as scavenging seabirds are opportunistic, and a gradual reduction in the availability of discards enables them to switch prey species and habituate to a reduction in food availability (Fondo et al. 2015).
Marine tourist boat operators conducting wildlife viewing trips off the coastline of Australia and other parts of the world may throw 'chum' (such as frozen squid) to attract seabirds, particularly albatrosses, to the vessel. This technique is used to provide tourists with the opportunity to see flocks of seabirds feeding and competing at a close range. This practice offers another artificial food source for the birds, and further encourages and habituates them to follow boats which in turn increases the likelihood of seabirds interacting with fishing vessels. In many cases, ‘chumming’ is carried out by experienced birdwatchers and is considered acceptable behaviour by some parts of the birding fraternity (for example, Onley & Schofield 2007), but not by others (for example, Orams 2002). Compared to discards from commercial fishing operations, ‘chumming’ is not a significant food source for albatrosses and petrels in Australian waters.
Marine infrastructure interactions
Threats from interactions with offshore installations and ships, including artificial lighting. |
Seabirds are at risk of disorientation at night from artificial light sources (Bruderer et al. 1999, Rodríguez et al. 2014, 2017). There is significant commercial, tourism, fishing, recreational vessel activity worldwide in coastal and offshore waters. Sea installations, particularly for energy extraction purposes including oil and gas, and wind farms are commonplace, particularly on continental shelf margins. Seabird interactions have been reported across the marine infrastructure sectors including vessels (Black 2005), oil and gas platforms (Wiese et al. 2001, Ronconi et al. 2015) and offshore wind farms (Dierschke et al. 2016). The response of seabirds to marine infrastructure can lead to avoidance behaviours, collisions where the bird may be killed or injured, and fallouts where the bird may be unable to return to the air without assistance (Commonwealth of Australia 2019b).
The development of offshore wind farm turbine infrastructure is an emerging issue, with offshore wind farm proposals being considered for sites along the southern Australian coastline within the foraging range of albatrosses and petrels.
Climate variability and change
Threats from climatic changes resulting in significant weather changes beyond historical variance, with effects on food dispersion and availability. |
Climate change is causing major shifts in the distribution of species throughout the world (Albouy et al. 2014). Seabirds including albatrosses and petrels are susceptible to extreme climatic events (Chambers et al. 2011, 2014, Rodríguez et al. 2019). The effects of climate change on seabirds in Australian marine ecosystems include ocean warming and changes in marine currents that have the potential to affect foraging patterns due to changes in marine productivity (Thomson et al. 2015, Cleeland et al. 2019), increased frequency of storm events (Walsh & Ryan 2000, McInnes et al. 2012) and changes in wind patterns affecting the energetic cost of foraging flights (Jouventin & Weimerskirch 1990, Marshall 2003).
A major effect of climate on seabirds will be changes in prey distribution (Robinson et al. 2005), particularly during the breeding season. As the world's oceans warm, the marine environments will lose oxygen, which is likely to increase metabolic pressure on marine species (Deutsch et al. 2015), including the prey species of albatrosses and petrels. Thus, habitable zones will shrink and some species are likely leave the upper water column (0-400 m) to seek refuge lower in the water column where cool, oxygenated waters still occur. This effect is expected to be largest near the equator, but contraction of habitable zones is also expected in the mid-latitudes where many fisheries occur, particularly in the northern hemisphere. Shifts by certain species towards higher latitudes may offset the habitat loss at least partially, however this move is likely to cause increased competition for polar and subpolar species (Deutsch et al. 2015). The effect of vertical shifts in prey species in the water column in response to higher sea surface temperatures, and any consequential reduction in the availability of such prey to albatrosses and petrels is presently unknown.
How seabirds will respond to climate change and whether or not they are capable of adapting to new conditions remains largely unknown (Thompson et al. 2015), and is likely to species-specific. The effect of climate change of the marine foraging ranges of albatrosses and petrels in Australia's jurisdiction is largely unknown, and poorly studied (Cleeland et al. 2019). As well, potential climate adaption strategies are limited to intervention options that may be taken at breeding sites (for example, Alderman & Hobday 2017).
Table 4 summarises the terrestrial and marine threats affecting the albatross and petrel species included in the recovery plan. The information in Table 4 is derived from the comprehensive species profile including risk matrices for each of the albatross and petrel species included in the recovery plan, which can be found at Appendix A.
Table 4: Terrestrial and marine threats affecting the albatross and petrel species included in the recovery plan, adapted from Salafsky et al. (2008) and Dias et al. (2019)
Threat description | Number of affected species and prioritisation within Australia's jurisdiction | Number of affected species and prioritisation beyond Australia's jurisdiction |
Terrestrial | | |
1. Human disturbance
Threats from human disturbance at or adjacent to breeding sites including direct habitat destruction, damage and disturbance, as well as interactions with built structures and artificial lighting. | Low (one species) | Low (2 species) |
Moderate (2 species) | Moderate (7 species) |
| |
| |
2. Introduced invasive species
Threats from invasive non-native species including predation, direct habitat destruction, degradation, damage and disturbance. | | |
| Moderate (8 species) |
| High (1 species) |
| Very High (8 species) |
3. Competition with native species Threats from competition with native wildlife including direct damage to nesting habitat and predation. | | |
| Moderate (4 species) |
| |
Very High (one species) | |
4. Disease Threats from native and non-native pathogens. | | Low (5 species) |
| Moderate (one species) |
High (one species) | High (2 species) |
| Very High (one species) |
5. Geological processes Threats from volcanic activity or earthquakes, including tsunamis and landslips. | Low (one species) | |
Moderate (2 species) | |
| |
| |
6. Climate variability and change Threats from climate change resulting in significant weather changes beyond historical variance, with effects on life history, breeding behaviour and success, breeding condition, and disease prevalence. | Low (2 species) | Low (4 species) |
Moderate (one species) | Moderate (2 species) |
High (one species) | High (3 species) |
Very High (one species) | Very High (one species) |
Threat description | Number of affected species and prioritisation within Australia's jurisdiction | Number of affected species and prioritisation beyond Australia's jurisdiction |
Marine threats | | |
1. Fisheries interactions and bycatch
Threats from interactions between seabirds and fishing gear. | — | — |
Moderate (2 species) | Moderate (3 species) |
— | — |
Very High (11 species) | Very High (20 species) |
2. Deliberate take
Threats from intentional killing of adults and juveniles. | — | Low (one species) |
— | — |
— | — |
3. Marine pollution
Threats from marine pollution, contamination and debris including plastics and microplastics. | — | — |
Moderate (15 species) | Moderate (15 species) |
— | — |
— | — |
4. Competition with fisheries for prey species
Threats from competition with fisheries for prey species. | — | — |
— | — |
— | — |
— | — |
5. Dependence on fisheries discards
Threats from reliance on fisheries discards. | Low (one species) | Low (one species) |
— | — |
— | — |
— | — |
6. Marine infrastructure interactions
Threats from interactions with offshore installations and ships, including artificial lighting. | — | — |
— | — |
— | — |
— | — |
7. Climate variability and change
Threats from climate change resulting in significant weather changes beyond historical variance, with effects on food dispersion and availability. | — | — |
Moderate (6 species) | Moderate (5 species) |
— | — |
Very High (one species) | Very High (one species) |
The populations of albatross and petrel species breeding and/or foraging in Australia’s jurisdiction have increased to such a size that each species no longer qualifies for listing as threatened under any of the Environment Protection and Biodiversity Conservation Act 1999 (Cth) listing criteria.
The objective of the recovery plan is:
To improve the conservation status of albatrosses and petrels so that these species are on a trajectory towards no longer being threatened in Australia's jurisdiction.
The success or otherwise of the recovery plan including the trajectory of species will be measured according to progress on the plan’s strategies and overarching actions. The objective will be achieved if within three generations (60 years approx.) there is a measurable and sustained positive population trend (compared to 2021 baseline counts) in the number of mature individuals within the Australian breeding populations of albatross and petrel species within the recovery plan. The recovery plan includes strategies and overarching actions to be applied within Australia’s jurisdiction that protect albatross and petrel breeding habitats, address threats to the conservation of the species on land and at sea, generate new knowledge to guide recovery, and increase public awareness. The plan will also assist in the implementation of Australia’s international environmental responsibilities, in particular to give effect to obligations under the Agreement on the Conservation of Albatrosses and Petrels and Convention on Biological Diversity, and including engagement with relevant regional conservation and fisheries organisations and arrangements to advance the conservation of albatrosses and petrels.
While it is not expected that the recovery plan objective will be reached in full within the timeframe of this plan, the following strategies are developed for the period of this plan to focus action towards achieving the objective and long-term vision:
- Ensure ongoing protection of albatross and petrel breeding sites and habitats in Australia's jurisdiction.
2. Improve the understanding of the size, structure and population trends for albatrosses and petrels breeding in Australia's jurisdiction.
3. Improve effectiveness of management measures that reduce land-based threats to albatrosses and petrels breeding in Australia's jurisdiction.
4. Improve effectiveness of management measures that reduce marine-based threats to albatrosses and petrels foraging in Australia's jurisdiction.
5. Improve understanding of generalised threats to albatrosses and petrels breeding and foraging within Australia's jurisdiction.
6. Improve community awareness of the conservation of albatrosses and petrels.
7. Achieve substantial progress towards global conservation of albatrosses and petrels in international conservation and fishing forums.

Actions identified for the recovery of albatrosses and petrels are described below. All actions apply to all species except those species-specific actions identified in Tables 6-12. While the overarching objective is long-term and is not likely be achieved prior to the scheduled five-year review of the recovery plan, or the 10-year life of the plan, indicators that may result in higher recruitment and increased population size, such as numbers of breeding birds and breeding success ascertained through monitored sites, will help to demonstrate any changes to vital rates during a shorter, five to 10-year timeframe.
Priorities assigned to actions should be interpreted as follows:
Priority 1: | Taking prompt action is necessary to mitigate the key threats to albatrosses and petrels and also provide valuable information to help identify long-term population trends. |
Priority 2: | Action would provide a more informed basis for the long-term management and recovery of the albatrosses and petrels. |
Priority 3: | Action is desirable, but not critical to the recovery of the albatrosses and petrels or assessment of trends in that recovery. |
During the life of this recovery plan new information will become available. This may include the emergence of new threats or changes in relative risk, changes in conservation status, or increased knowledge about a threat. As new information becomes available it will be taken into consideration in the context of this plan.
The overarching actions in this recovery plan are designed to assess and address threats to the recovery of albatrosses and petrels in Australia's jurisdiction, and to enable and ensure the recovery of the species (Table 5). These have been devised to deliver tangible benefits to meet the recovery plan strategies. It is expected that every action will be progressed or completed during the life of this plan.
Table 5: Summary of overarching actions in the National Recovery Plan for albatrosses and petrels.
Overarching actions | Strategy to achieve objective |
A. | Assessing and addressing threats | |
A1 | Ongoing protection of albatross and petrel species breeding sites and habitats in Australia's jurisdiction | 1 |
A2 | Prevent introduction of alien species to breeding islands in Australia's jurisdiction | 3 |
A3 | Identify whether competition with native species is causing population declines | 3 |
A4 | Identify diseases likely to have a population-level effect on breeding populations | 3 |
A5 | Avoid or minimise incidental catch (or bycatch) of seabirds during fishing operations in Australia's jurisdiction | 4 |
A6 | Advocate for effective international measures for conserving albatrosses and petrels | 7 |
A7 | Minimise the effects of marine debris, plastics and pollution | 5 |
B. | Enabling and measuring recovery | |
B1 | Monitor population and conservation status of breeding populations in Australia's jurisdiction | 2 |
B2 | Monitor the effects of fishing on albatrosses and petrels in Australia's jurisdiction | 4 |
B3 | Increase community understanding of and involvement in the conservation of albatrosses and petrels | 6 |
B4 | Increase understanding of the effects of climate change on albatrosses and petrels in Australia, and identify ways to increase the resilience of the species to these effects. | 5 |
B5 | Implement statutory requirements | 6 |
The cost estimates shown below against each strategy are provided for guidance. They do not represent funding commitments.
Table 6: Strategy 1: Actions to ensure ongoing protection of albatross and petrel breeding site habitats in Australia's jurisdiction.
Overarching Action | Actions | Priority | Performance criteria | Responsible agencies and potential partners | Indicative cost |
A1 Ongoing protection of albatross and petrel breeding sites and habitats in Australia's jurisdiction. | 1a_Rigorous biosecurity measures are implemented to reduce the risk of introduction of invasive species. 1b_Human disturbance is managed through limited access at breeding sites. 1c_Assess the benefits of current and potential listings of albatross and petrel habitats on the Register of Critical Habitat. | 1 | Monitoring at all breeding sites of albatrosses and petrels in Australia's jurisdiction demonstrates adequate protection measures are in place. Critical habitat listings for albatrosses and petrels follow contemporary listing modalities. | Australian Government State Governments | $20 000 pa |

Table 7: Strategy 2: Actions to improve the understanding of the size, structure and population trends for albatrosses and petrels breeding in Australia’s jurisdiction.
Overarching Action | Actions | Priority | Performance criteria | Responsible agencies and potential partners | Indicative cost |
B1 Monitor population and conservation status of breeding populations of albatrosses and petrels in Australia's jurisdiction. | 2a_Breeding populations are monitored in line with the following timeframes, subject to logistical constraints: - each year: Macquarie Island albatrosses and petrels, Tasmanian Shy Albatross (Albatross Island, Pedra Branca, and the Mewstone)
- at least every 2 years: AAT Southern Giant Petrel (Giganteus, Hawker and Frazier Islands)
- at least every 5 years: Bishop and Clerk Islets Black-browed Albatross
- at least every 5 years: Heard Island albatrosses and petrels
- at least every 10 years: McDonald Islands' albatrosses and petrels.
2b_Obtain population dynamics data for each breeding population where feasible, in line with desired monitoring timeframes, including: - conducting long-term demographic studies
- identifying driving factors for observed population changes
- identifying overlap of foraging range with fisheries
- identifying population trends and changes in these over time
- quantifying scale and nature of dietary requirements
- assessing effect of offal discharge on reproductive success.
2c_Identify options to expand the range of innovative, cost-effective techniques for collecting and analysing population trend data and demographic data for populations. 2d_Contribute to national and global dissemination of population and conservation status data. | 1 | Gaps in demographic data are identified for each breeding population. Sufficient data are made available through a purpose-built framework that enables priority conservation management decision-making. | Australian Government Tasmanian Government Research community | $2 250 000 (over life of the plan) |
Table 8: Strategy 3: Actions to improve effectiveness of management measures that reduce land-based threats to albatrosses and petrels breeding within Australia’s jurisdiction.
Overarching Action | Actions | Priority | Performance criteria | Responsible agencies and potential partners | Indicative cost |
A2 Prevent introduction of pest species to albatross and petrel breeding islands | 3a_Effective pre-arrival quarantine measures are in place and implemented for any visits to breeding islands. 3b_Monitoring is occurring at breeding islands for presence of introduced species. | 1 | Monitoring at breeding islands for presence of introduced species uses a risk-based approach. A rapid response strategy has been developed (and implemented) for each breeding island where threatened albatrosses and petrels are likely to occur. | Australian Government Tasmanian Government Tourism sector | $125 000 (over life of the plan) |
A3 Identify whether competition with native species is causing a population level decline in the numbers of Shy Albatross at Pedra Branca. | 3c_Determine the nature of competition between Shy Albatross and Australasian Gannet on Pedra Branca, subject to logistical constraints affecting access at this site. 3d_Undertake a gap analysis and risk assessment to determine the viability of translocation options for the affected population, as well as albatrosses and petrels generally. | 1 | Risk based options are developed for reducing levels of inter-species competition where this competition affects the long-term viability of the breeding site. Cost-effective measures are implemented, as appropriate, to reduce levels of inter-species competition at the breeding site. Gap analysis and risk assessment determine the viability of translocation options. | Australian Government Tasmanian Government Research community | $125 000 (over life of the plan) |
A4 Identify diseases likely to have a population level effect on breeding populations. | 3e_Undertake a gap analysis and risk assessment to determine the level of risk of disease introductions and effects on breeding islands. 3f_Develop, and where appropriate, implement a strategy for mitigating risk of population level effects of disease on breeding populations. | 2 | A risk-based strategy for mitigating risk of population level effects of disease on breeding populations is in place. Measures to mitigate the risk of population level effects of disease on breeding populations are implemented. | Australian Government Tasmanian Government Research community | $125 000 (over life of the plan) |
Table 9: Strategy 4: Actions to improve effectiveness of management measures that reduce marine based threats to albatrosses and petrels foraging in Australia's jurisdiction.
Overarching Action | Actions | Priority | Performance criteria | Responsible agencies and potential partners | Indicative cost |
A5 Avoid or minimise bycatch of albatrosses and petrels during fishing operations in Australia's jurisdiction. | 4a_Promote best practice seabird mitigation measures in line with ACAP's relevant best practice advice. 4b_Quantify the risk of seabird bycatch during fishing operations and develop options to reduce this risk. 4c_Establish effective cooperation between the Recovery Team and TAP Stakeholder Group to support development of options to reduce seabird bycatch in fisheries. | 1 | The Threat Abatement Plan - Seabirds and the National Plan of Action – Seabirds are implemented for fishing operations in Australian waters. The risk of seabird bycatch during fishing operations has been quantified and options to reduce this risk implemented. Options for avoiding or minimising offal discharge have been developed, particularly in longline and trawl fisheries. | Australian Government State Governments Fishing Industry Research community | $150 000 pa |
B2 Monitor effects of fishing on albatrosses and petrels in Australia's jurisdiction. | 4d_Monitor frequency of fishing equipment ingestion / entanglement at breeding colonies. 4e_Encourage development of fisheries assessments that incorporate information about diet composition of albatross and petrel species. 4f_Encourage the uptake and use of artificial intelligence systems that enable seabird bycatch identification. | 1 | Collection of seabird species bycatch data accurately reflects bycatch to species level particularly for albatrosses and petrels, in commercial and recreational fisheries. The causes of seabird bycatch and approaches to mitigate seabird bycatch is improved through the use of artificial intelligence systems that identify bycaught seabird species to species level. | Australian Government State Governments Fishing Industry Research community | $50 000 pa |
Table 10: Strategy 5: Actions to improve understanding of generalised threats to albatrosses and petrels breeding and foraging within Australia's jurisdiction.
Overarching Action | Actions | Priority | Performance criteria | Responsible agencies and potential partners | Indicative cost |
B4 Improve understanding of the effects of climate change on albatrosses and petrels, and identify ways to increase the resilience of the species to these effects. | 5a_Undertake research and monitoring into the effects of climate change on albatrosses and petrels breeding and foraging within Australia's jurisdiction. | 1 | Research and monitoring inform management actions that contribute to increased resilience of albatrosses and petrels in response to future climate scenarios. | Australian Government State Governments Fishing Industry Research community Environmental NGOs | $50 000 pa |
A7 Improve understanding of and reduce the effects of marine debris, plastics and pollution on albatrosses and petrels. | 5b_Undertake, as feasible, monitoring of breeding colonies for marine debris, plastics and marine pollution impacts including, as a priority: - incidence of oiled birds at nest
- levels of marine debris egestion and entanglement at nest
- effect of plastics and marine pollution
- develop baseline measures of levels of heavy metals and persistent organic pollutants.
5c_Risk based response strategies for marine pollution incidents are developed. | 2 | The Threat Abatement Plan – Marine Debris is implemented with particular focus on actions where seabirds are identified. Risk-based response strategies are implemented where appropriate, for marine pollution incidents that have the potential to affect breeding populations. | Australian Government State Governments Local Government Fishing Industry Research community Environmental NGOs | $50 000 pa |
Table 11: Strategy 6: Actions to improve community awareness of the conservation of albatrosses and petrels.
Overarching Action | Actions | Priority | Performance criteria | Responsible agencies and potential partners | Indicative cost |
B5 Establish and maintain a Recovery Team for threatened albatrosses and petrels. | 6a_The Recovery Team meets at least annually and coordinates, reviews and reports on recovery outcomes. | 3 | Recovery Team established with terms of reference approved within six months of making the recovery plan. | All | $5 000 pa |
B5 Review the recovery plan within five years after making. | 6b_Undertake a five-year review of recovery plan including consultation with stakeholders. | 1 | Completion of five-year review of recovery plan including consultation with stakeholders and the public, within statutory requirements and timeframes. | Australian Government State Governments Fishing Industry Research community Environmental NGOs | $50 000 (over the life of the plan) |
B3 Develop and implement a broad strategy to raise awareness and educate the public and industry about albatross and petrel conservation. | 6c_Information and Guidelines are developed that support and encourage the fishing community to be involved in mitigating the effects of fishing on seabirds. 6d_Conduct community awareness raising initiatives concerning albatross and petrel conservation, including threats and recovery actions particularly in support of Threatened Species Day and World Albatross Day initiatives. | 2 | Information and guidelines effectively inform, support and encourage fishers to be involved in mitigating the effects of fishing on seabirds. Fishers and fishing industry are engaged in process. Industry and community awareness raising initiatives concerning albatross and petrel conservation, including threats and recovery actions have been undertaken. | Australian Government State Governments Fishing Industry Research community Environmental NGOs | $25 000 pa |
B3 Support use of citizen science in the conservation of albatrosses and petrels. | 6e_Develop and communicate information that encourages citizen scientists' involvement in albatross and petrel conservation. | 3 | Information and guidelines inform and encourage citizen scientists' involvement in albatross and petrel conservation. | Environmental NGOs including BirdLife Australia Tourism Industry | $15 000 pa |
Table 12: Strategy 7: Actions to achieve substantial progress towards global conservation of albatrosses and petrels in international conservation and fishing forums.
Overarching Action | Actions | Priority | Performance criteria | Responsible agencies and potential partners | Indicative cost |
A6 Advocate for effective international measures for conserving albatrosses and petrels. | 7a_A strategy is developed on priorities for international seabird conservation advocacy. 7b_Undertake to advocate for best practices for reducing seabird bycatch and effective monitoring of seabird bycatch in international fisheries and conservation forums including as a priority: - mandatory electronic monitoring of fishing operations
- mandatory reporting of seabird mortalities to species level including through use of artificial intelligence systems reporting of seabird mitigation measures employed.
7c_Undertake to advocate for ongoing action to combat illegal, unreported and unregulated fishing in international fisheries and conservation forums due to the lack of seabird bycatch mitigation in these fisheries. 7d_Encourage through diplomatic and other means Range States to cooperate to conserve albatrosses and petrels. | 1 | The design and implementation of education strategies and materials for fishers and the community demonstrate support for and awareness of the conservation needs of albatrosses and petrels. Australia's engagement in relevant international forums promotes uptake of improved seabird mitigation measures and seabird bycatch reporting outcomes. | Australian Government ACAP Parties State Governments Fishing Industry Research community Environmental NGOs | $150,000 pa |
A6 Avoid or minimise bycatch of albatrosses and petrels during fishing operations involving Australian vessels operating beyond Australia's jurisdiction. | 7e_Ensure high seas fishing operations by Australian vessels implement: - mandatory electronic monitoring of fishing operations
- mandatory reporting of seabird mortalities to species level including through use of artificial intelligence systems
- mandatory reporting of seabird mitigation measures employed
- promotion of best practice seabird mitigation measures in line with ACAP's relevant best practice advice.
| 1 | The Threat Abatement Plan – Seabirds and the National Plan of Action – Seabirds are implemented for fishing operations on the high seas by Australian vessels. The Australian fishing industry is actively engaged and supportive of improved seabird mitigation measures and seabird bycatch reporting outcomes for high seas fishing operations. | Australian Government Fishing Industry Research community | $150 000 pa |
The performance of this recovery plan will be considered at the completion of the plan. The performance of the plan will be rated against how successful the plan has been in meeting its overarching actions (Table 13) and will give an indication of the degree of progress towards the long-term recovery objective. The approach to reviewing performance under the Recovery Plan will be developed in consultation with the Recovery Team established under this plan, and the Threatened Species Scientific Committee. The progress of the plan will be reported at a five year (mid-term) review of the plan.
Table 13: Performance measures.
Performance rating for the recovery plan | Overarching actions | Progress towards long-term recovery objective |
Successful | All met | Excellent |
Moderately successful | Eight of the 12 overarching actions met | Sound |
Moderately unsuccessful | Six of the 12 overarching actions met | Adequate |
Unsuccessful | Fewer than five overarching actions met | Failure |

Recovery teams provide advice and assist in coordinating actions described in recovery plans. They include representatives from organisations with a direct interest in the recovery of the species, including those involved in funding and those participating in actions that support the recovery of the species. The recovery of threatened albatrosses and petrels is coordinated by the Albatross and Petrel Recovery Team that has the responsibility of providing advice, coordinating and directing the implementation of the recovery actions outlined in this recovery plan. The membership of the Recovery Team, which may change over time, currently includes individuals within relevant government agencies, the fishing industry, environmental non-government organisations, and expertise from independent researchers.
To contact the Recovery Team please email: AlbatrossPetrelRecoveryTeam@aad.gov.au.

The recovery process will not be achieved prior to the scheduled five-year review or within the 10-year life of this recovery plan. A recovery plan should therefore remain in place until such time as the breeding and/or foraging populations of albatrosses and petrels in Australia's jurisdiction have improved to the point at which these species no longer meet threatened species status under the EPBC Act.
The cost of implementation of this recovery plan should be incorporated into the core business expenditure of the affected organisations and through additional funds obtained for the explicit purpose of implementing the plan. It is expected that Commonwealth and state agencies will use this plan to prioritise actions to protect the species and enhance their recovery, and that projects will be undertaken according to agency priorities and available resources. To maximise the conservation outcomes and cost effectiveness of this plan, it is intended that the recovery actions proposed complement, where possible, those of other protected matters.
The summary of cost estimates against each strategy shown in Table 14 below are provided for guidance and do not represent funding commitments.
Table 14: Summary of recovery actions and estimated costs for the first five years of implementation (these estimated costs do not take into account inflation over time).
Action | Cost |
Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total |
Strategy 1 | $20,000 | $20,000 | $20,000 | $20,000 | $20,000 | $100,000 |
Strategy 2 | $250,000 | $250,000 | $250,000 | $1,750,000* | $250,000 | $2,750,000 |
Strategy 3 | $75,000 | $75,000 | $75,000 | $75,000 | $75,000 | $375,000 |
Strategy 4 | $200,000 | 200,000 | $200,000 | $200,000 | $200,000 | $1,000,000 |
Strategy 5 | $200,000 | $200,000 | $200,000 | $200,000 | $200,000 | $1,000,000 |
Strategy 6 | $45,000 | $45,000 | $45,000 | $45,000 | $95,000 | $275,000 |
Strategy 7 | $300,000 | $300,000 | $300,000 | $300,000 | $300,000 | $1,500,000 |
TOTAL | $1090,000 | $1,090,000 | $1,090,000 | $2,590,000 | $1,140,000 | $7,000,000 |
* The increase in estimated expenditure in Year 4 under Strategy 2 is associated with an anticipated management visit to Heard Island.
Reducing marine and terrestrial anthropogenic impacts affecting threatened albatrosses and petrels will likely benefit seabirds generally including other listed threatened, migratory and marine species over time, particularly:
- storm petrel species within the Family Oceanitidae and Family Hydrobatidae
- petrel, fulmar, prion, shearwater, and diving petrel species within the Family Procellariidae
- gannet species within the Family Fregatidae
- skua species within the Family Stercorariidae
- penguin species within the Family Spheniscidae.
Many of these species breed at the same locations and/or forage across similar ranges as the albatross and petrel species included in this recovery plan (Parks and Wildlife Service 2006, Commonwealth of Australia 2014, 2018a, 2018b). Implementation of the plan will also have positive outcomes for other marine and coastal species and listed ecological communities through improving habitat quality.
This recovery plan is not linked directly to any Australian Government funding programs. However, the plan helps direct the focus of government funding programs to activities that will help to meet identified actions. While the Australian Government is unable to provide funding to cover all actions in this plan, it is committed to implement the plan to the extent to which it applies in Commonwealth areas. Investment in addressing the threats to the conservation of albatrosses and petrels will be further determined by the level of resources that the fishing industry, government and non-government stakeholders are also able to commit to implementation of the actions under the plan.
The successful implementation of the actions under this recovery plan will rely on support from stakeholders. Partnerships involving the fishing industry, government and non-government organisations, universities, community groups and Indigenous groups will be key to successfully delivering the actions under the plan.
The plan does not of itself impose any economic burdens on fishers, rather it supports the implementation of related threat abatement plans (Commonwealth of Australia 2018a, 2018b) and the NPOA-Seabirds (DAWR 2018). However, the imposition of technical and operational mitigation measures may have financial impacts on fishers. These may be partially offset, for example, if measures to avoid or minimise interactions with seabirds result in a market advantage for fish marketed as being caught in a manner that avoids seabird bycatch.
While wildlife tourism attracts many visitors (Higginbottom 2004), with bird watching activities among seabird colonies being particularly attractive tourist destinations (for example, Yorio et al. 2001), this activity along with recreational boating and fishing may disturb albatrosses and petrels at their breeding sites, as well as foraging birds adjacent to these locations. Restrictions on access to albatross and petrel breeding sites in Australia have been in place under previous recovery plans and do not generally impact landowners, land managers and developers. Albatrosses and petrels are found on remote, offshore island locations, and access to individual albatross and petrel populations is restricted by significant logistical difficulties. These sites are, therefore, relatively less attractive for ecotourism and other visitation, when compared to mainland bird colonies. Biosecurity protocols applying to research activities helps minimise the risk of introduction of pest species, and thereby avoid the need for future pest eradication programs, as was necessary to eradicate cats, rabbits, rats and mice from Macquarie Island (Robinson & Copson 2014, Alderman et al. 2019).
There is potential for social benefits to flow from community members engaging in citizen science concerning albatrosses and petrels. For example, a small, dedicated group has been conducting routine surveys of offshore seabird abundance in New South Wales waters for nearly 20 years (Gorta et al. 2019). This citizen science provides a long-term data set that has helped to assess whether the abundance of particular albatross and petrel populations foraging off south-eastern Australia has changed over time.
Many marine species have cultural significance to Aboriginal and Torres Strait Islander people. There is some evidence from Aboriginal language and history of a link to albatross and their breeding habitat in Tasmania. The Tasmanian Aboriginal people of the north-west region called Albatross Island tangatema and they may have visited the island by canoe in calm conditions when the journey between Hunter and Albatross Islands could be safely negotiated (Wastell et al. 2015) and the island has also been referred to as namanu rruni (Alderman 2018, pers. comm., 5 September 2018).

Organisations likely to be affected by the actions proposed in this recovery plan include: international multilateral and bilateral agreements, government agencies (Commonwealth and state), particularly those involved with environmental protection, conservation and fisheries management programs, the fishing industry, Indigenous community groups, researchers, and conservation groups. This list however should not be considered exhaustive, as there may be other interest groups that would like to be included in the future or need to be considered when specialised tasks are required.
Table 15 lists some of the interest groups, how they could contribute to the success of the recovery plan and the potential benefits/impacts that may emerge from the plan’s implementation.
Table 15: Affected interests.
Interest Group | Contribution | Impacts/Benefits |
Australian Government | Responsible for development, coordination and evaluation of the plan Responsible for implementation of the plan in Commonwealth areas Providing financial support for implementation of the plan Education | Informed decision making concerning EPBC Act requirements Greater ability to deliver on domestic and international obligations with regard to albatross and petrel conservation Increased knowledge of seabirds and their habitats – increased exchange of information between decision-makers and the community |
State government agencies | Contributing to the development of the plan and its implementation within jurisdictional boundaries Tasmania: Providing expert advice on albatrosses and petrels and their biology, ecology and conservation Tasmania: Contributing to the implementation and evaluation of the plan, particularly in conducting research and monitoring programs for breeding species – implementing on ground activities | Greater ability to deliver on state obligations with regard to albatross and petrel conservation Increased knowledge of seabirds and their habitats – increased exchange of information Tasmania: Monitoring of albatross and petrel breeding populations and success of conservation actions |
Fishing industry | Contributing to the development and evaluation of the plan, particularly in marine settings Contributing to development of innovative conservation outcomes for industry | Greater ability to deliver on Commonwealth and state obligations with regard to albatross and petrel conservation Increased knowledge of seabirds and their habitats – increased exchange of information |
Indigenous community groups | Contributing traditional knowledge | Increased knowledge of albatrosses and petrels, and their habitats – increased exchange of information |
Conservation Groups | Contributing to the implementation and evaluation of the plan, particularly in conducting education and research programs | Opportunity to seek funding for conservation and awareness projects under biodiversity conservation programs Delivering on charitable/not-for-profit goals benefiting the public |
Australasian Seabird Group / BirdLife Australia | Providing expert advice on albatrosses and petrels and their biology, ecology and conservation Contributing to the implementation and evaluation of the plan Education | Opportunity to seek funding for conservation and awareness projects under biodiversity conservation programs Greater coordination of targeted conservation projects |
Community and Special Interest groups | Contributing to the plan and volunteering for conservation and awareness activities – implementing on ground activities | More albatrosses and petrels to enjoy Opportunity to participate in conservation projects |
Researchers | Contributing to the implementation and evaluation of the plan | Increased exchange of information – opportunity to seek funding for research Opportunity to establish collaborations within Australia and internationally |
The recovery plan has been developed through extensive consultation with a broad range of stakeholders. Consultation included representatives from government agencies, fishing industry, environmental non-government organisations, research organisations, and Indigenous interest groups. Notice of the draft plan was made available for public comment for three months between 21 May 2021 and 27 August 2021. Any comments received that were relevant to the recovery of threatened albatrosses and petrels were considered by the Threatened Species Scientific Committee as part of the development of this plan.
Section 279 of the EPBC Act provides for the review of action under a recovery plan at any time and requires that each plan be reviewed at intervals no greater than five years from when it was endorsed and made publicly available. The review of this recovery plan will determine the performance of the plan and will be coordinated by the Department of Climate Change, Energy, the Environment and Water in association with relevant Australian and state government agencies, and key stakeholder groups, such as the fishing industry, environmental non-governmental organisations, research organisations, and Indigenous interest groups. The review will examine action under the plan and assess progress towards achieving the plan's objective. The review’s recommendations will form the basis of a revised plan, if required.
Key stakeholders who may be involved in the review of the performance of the recovery plan, include organisations likely to be affected by the actions proposed in this plan and are expected to include:
Australian Government
Australian Fisheries Management Authority
Department of Climate Change, Energy, the Environment and Water
Threatened Species Commissioner
Threatened Species Scientific Committee
State governments
Department of Biodiversity, Conservation and Attractions (WA)
Department of Environment and Water (SA)
Department of Environment, Land, Water and Planning (Vic)
Department of Natural Resources and Environment (Tas)
Department of Planning and Environment (NSW)
Department of Environment and Science (Qld)
Fishing industry
Representative groups, and individual fishers and companies operating in Commonwealth and State-managed commercial and recreational fisheries.
Non-government organisations
Antarctic and Southern Ocean Coalition
Australasian Seabird Group
BirdLife Australia
Humane Society International
World Wide Fund for Nature
Indigenous interest groups
Tasmanian Aboriginal Centre
Institutions
Universities and other research organisations
Regular annual meetings of the newly established Recovery Team will help ensure that progress is monitored and implementation of the recovery plan is progressed.
ABARES (Australian Bureau of Agricultural and Resource Economics and Sciences ABARES) (2021) Fishery status reports 2021. Available on the internet at: https://www.agriculture.gov.au/abares/research-topics/fisheries/fishery-status
Abbott CL, Double MC, Gales R, Baker GB, Lashko A, Robertson CJR & Ryan PG (2006a) Molecular provenance analysis for shy and white-capped albatrosses killed by fisheries interactions in Australia, New Zealand, and South Africa. Conservation Genetics 7, 531-542.
Abbott CL, Double MC, Gales R & Cockburn A (2006b) Copulation behaviour and paternity in shy albatrosses (Thalassarche cauta). Journal of Zoology 270, 628-635.
Abraham E R (2010) Warp strike in New Zealand trawl fisheries, 2004-05 to 2008-09. New Zealand Aquatic Environment and Biodiversity Report No. 60. Dragonfly, Wellington, New Zealand.
Abraham E, Richard Y, Walker N, Gibson W, Ochi D, Tsuji S, Kerwath S, Winker H, Parsa M, Small C, Waugh S (2019) Assessment of the risk of surface longline fisheries in the Southern Hemisphere to albatrosses and petrels, for 2016. Commission for the Conservation of Southern Bluefin Tuna, 13th Meeting of the Ecologically Related Species Working Group, 28-31 May 2019, Canberra, CCSBT-ERS/1905/17.
ACAP (Agreement on the Conservation of Albatrosses and Petrels) (2006) Report of the Second Session of the Meeting of the Parties. Agreement on the Conservation of Albatrosses and Petrels. Second Meeting of the Parties, 13-17 November 2006, Christchurch, New Zealand, MoP2 Report.
ACAP (2012a) Species assessments: Amsterdam Albatross Diomedea amsterdamensis. Version 19 September 2012. Available on the internet at: http://www.acap.aq/en/acap-species/233-amsterdam-albatross/file.
ACAP (2012b) Species assessments: Antipodean Albatross Diomedea antipodensis. Version 19 September 2012. Available on the internet at: http://www.acap.aq/en/acap-species/289-antipodean-albatross/file.
ACAP (2012c) Species assessments: Atlantic Yellow-nosed Albatross Thalassarche chlororhynchos. Version 19 September 2012. Available on the internet at: http://www.acap.aq/en/acap-species/290-atlantic-yellow-nosed-albatross/file.
ACAP (2012d) Species assessments: Black-browed Albatross Thalassarche melanophris. Version 19 September 2012. Available on the internet at: http://www.acap.aq/en/acap-species/238-black-browed-albatross/file.
ACAP (2012e) Species assessments: Black Petrel Procellaria parkinsoni. Version 19 September 2012. Available on the internet at: https://www.acap.aq/en/acap-species/291-black-petrel/file
ACAP (2012f) Species assessments: Buller's Albatross Thalassarche bulleri. Version 19 September 2012. Available on the internet at: http://www.acap.aq/en/acap-species/292-buller-s-albatross/file.
ACAP (2012g) Species assessments: Campbell Albatross Thalassarche impavida. Version 19 September 2012. Available on the internet at: http://www.acap.aq/en/acap-species/293-campbell-albatross/file.
ACAP (2012h) Species assessments: Chatham Albatross Thalassarche eremita. Version 19 September 2012. Available on the internet at: http://www.acap.aq/en/acap-species/294-chatham-albatross/file.
ACAP (2012i) Species assessments: Grey Petrel Procellaria cinerea. Version 19 September 2012. Available on the internet at: https://www.acap.aq/en/acap-species/249-grey-petrel/file.
ACAP (2012j) Species assessments: Grey-headed Albatross Thalassarche chrysostoma. Version 19 September 2012. Available on the internet at: http://www.acap.aq/en/acap-species/248-grey-headed-albatross/file.
ACAP (2012k) Species assessments: Indian Yellow-nosed Albatross Thalassarche carteri. Version 19 September 2012. Available on the internet at: http://www.acap.aq/en/acap-species/295-indian-yellow-nosed-albatross/file.
ACAP (2012l) Species assessments: Laysan Albatross Phoebastria immutabilis. Version 19 September 2012. Available on the internet at: http://www.acap.aq/en/acap-species/254-laysan-albatross/file.
ACAP (2012m) Species assessments: Light-mantled Albatross Phoebetria palpebrata. Version 19 September 2012. Available on the internet at: http://www.acap.aq/en/acap-species/255-light-mantled-albatross/file.
ACAP (2012n) Species assessments: Northern Giant Petrel Macronectes halli. Version 19 September 2012. Available on the internet at: http://www.acap.aq/en/acap-species/264-northern-giant-petrel/file.
ACAP (2012o) Species assessments: Northern Royal Albatross Diomedea sanfordi. Version 19 September 2012. Available on the internet at: http://www.acap.aq/en/acap-species/296-northern-royal-albatross/file.
ACAP (2012p) Species assessments: Salvin's Albatross Thalassarche salvini. Version 19 September 2012. Available on the internet at: http://www.acap.aq/en/acap-species/297-salvin-s-albatross/file.
ACAP (2012q) Species assessments: Shy Albatross Thalassarche cauta. Version 19 September 2012. Available on the internet at: http://www.acap.aq/en/acap-species/299-shy-albatross/file
ACAP (2012r) Species assessments: Sooty Albatross Phoebetria fusca. Version 19 September 2012. Available on the internet at: http://www.acap.aq/en/acap-species/287-sooty-albatross/file
ACAP (2012s) Species assessments: Southern Giant Petrel Macronectes giganteus. Version 19 September 2012. Available on the internet at: http://www.acap.aq/en/acap-species/288-southern-giant-petrel/file.
ACAP (2012t) Species assessments: Southern Royal Albatross Diomedea epomophora. Version 19 September 2012. Available on the internet at: http://www.acap.aq/en/acap-species/300-southern-royal-albatross/file.
ACAP (2012u) Species assessments: Tristan Albatross Diomedea dabbenena. Version 19 September 2012. Available on the internet at: http://www.acap.aq/en/acap-species/303-tristan-albatross/file.
ACAP (2012v) Species assessments: Wandering Albatross Diomedea exulans. Version 19 September 2012. Available on the internet at: http://www.acap.aq/en/acap-species/304-wandering-albatross/file.
ACAP (2012w) Species assessments: Westland Petrel Procellaria westlandica. Version 19 September 2012. Available on the internet at: https://www.acap.aq/en/acap-species/316-westland-petrel/file.
ACAP (2012x) Species assessments: White-capped Albatross Thalassarche steadi. Version 19 September 2012. Available on the internet at: http://www.acap.aq/en/acap-species/317-white-capped-albatross/file.
ACAP (2012y) Species assessments: White-chinned Petrel Procellaria aequinoctialis. Version 19 September 2012. Available on the internet at: https://www.acap.aq/en/acap-species/306-white-chinned-petrel/file.
ACAP (2013a) ACAP Breeding Site No. 18: Albatross Island, Bass Strait, home of Australia's endemic Shy Albatross. Available on the Internet at: https://www.acap.aq/news/news-archive/60-2013-news-archive/1343-acap-breeding-sites-no-18-albatross-island-bass-strait-home-of-australia-s-endemic-shy-albatross.
ACAP (2013b) ACAP Breeding Site No. 19: Macquarie Island, a recovery world heritage site. Available on the Internet at: https://www.acap.aq/news/news-archive/60-2013-news-archive/1347-breeding-sites-no-19-macquarie-island-a-recovering-sub-antarctic-world-heritage-site.
ACAP (2013c) ACAP Breeding Site No. 21: Heard Island, the only subantarctic island with an active volcano. Available on the Internet at: https://www.acap.aq/news/news-archive/60-2013-news-archive/1351-acap-breeding-sites-no-21-heard-island-the-only-sub-antarctic-island-with-an-active-volcano.
ACAP (2013d) ACAP Breeding Site No. 28: Mewstone, where Shy Albatross get vertical. Available on the Internet at: https://www.acap.aq/news/news-archive/60-2013-news-archive/1378-acap-breeding-sites-no-28-mewstone-where-shy-albatrosses-get-vertical.
ACAP (2013e) ACAP Breeding Site No. 29: Pedra Branca, where shy albatrosses compete for space with Australasian gannets. Available on the Internet at: https://www.acap.aq/news/news-archive/60-2013-news-archive/1382-acap-breeding-sites-no-29-pedra-branca-where-shy-albatrosses-compete-for-space-with-australasian-gannets.
ACAP (2013f) ACAP Breeding Site No. 43: McDonald Islands, Australia's least known and visited subantarctic locality. Available on the Internet at: https://www.acap.aq/news/news-archive/60-2013-news-archive/1486-acap-breeding-sites-no-43-mcdonald-islands-australia-s-least-known-and-visited-sub-antarctic-locality.
ACAP (2014) ACAP Breeding Site No. 66: Bishop and Clerk Islets—Australia's southernmost albatross colony. Available on the Internet at: https://acap.aq/en/latest-news/1684-acap-breeding-site-no-66-bishop-and-clerk-islets-australia-s-southernmost-black-browed-albatross-colony.
ACAP (2015) Seabird Bycatch Identification Guide. Updated August 2015. Available on the Internet at: https://acap.aq/en/resources/bycatch-mitigation/seabird-bycatch-id-guide.
ACAP (2019a) Report of the eleventh meeting of the Advisory Committee Agreement on the Conservation of Albatrosses and Petrels. 11th Meeting of the Advisory Committee, 13-17 May 2019, Florianópolis, Brazil, AC11 Report.
ACAP (2019b) Hook removal from seabirds guide. Available on the Internet at: https://www.acap.aq/resources/bycatch-mitigation/hook-removal-from-seabirds-guide.
ACAP (2021a) ACAP review and best practice advice for reducing the impact of demersal longline fisheries on seabirds. Agreement on the Conservation of Albatrosses and Petrels. 12th Meeting of the Advisory Committee, 31 August – 2 September 2021, online. Available on the Internet at: https://www.acap.aq/bycatch-mitigation/mitigation-advice.
ACAP (2021b) ACAP review and best practice advice for reducing the impact of pelagic and demersal trawl fisheries on seabirds. Agreement on the Conservation of Albatrosses and Petrels. 12th Meeting of the Advisory Committee, August – 2 September 2021, online. Available on the Internet at: https://www.acap.aq/bycatch-mitigation/mitigation-advice.
ACAP (2021c) ACAP review and best practice advice for reducing the impact of pelagic longline fisheries on seabirds. Agreement on the Conservation of Albatrosses and Petrels. 12th Meeting of the Advisory Committee, August – 2 September 2021, online. Available on the Internet at: https://www.acap.aq/bycatch-mitigation/mitigation-advice.
ACAP (2022) Report on the progress with implementation of the Agreement 2018-2021, Agreement on the Conservation of Albatrosses and Petrels, Seventh Meeting of the Parties, 9-13 May 2022, online, MoP7 Doc 10.
Adams NJ (1992) The distribution, population status and conservation of southern African seabirds. Stichting Greenpeace Council, Amsterdam.
AFMA (Australian Fisheries Management Authority) (2022a) Gillnets. Available on the Internet at: https://www.afma.gov.au/fisheries-management/methods-and-gear/gillnets.
AFMA (2022b) How we manage our fisheries. Available on the Internet at: https://www.afma.gov.au/about/how-fisheries-managed.
Agency for Toxic Substances and Disease Registry (2000) Toxicological profile for polychlorinated biphenyls (PCBs). United States Department of Health and Human Services, Public Health Service, Atlanta, GA.
Agnew DJ, Pearce J, Pramod G, Peatman T, Watson R, Beddington JR & Pitcher TJ (2009) Estimating the worldwide extent of illegal fishing. PLoS One 4: e4570.
Akkers T (2002) Research in the Southern Oceans, in Research highlights 2001–2002, Vol. 11. Department of Environmental Affairs and Tourism, Pretoria. pp. 78-82.
Albouy C, Velez L, Coll M, Colloca F, Le Loc’h F, Mouillot D & Gravel D (2014) From projected species distribution to food-web structure under climate change. Global Change Biology 20, 730-741.
Alderman RL (2012) The shy albatross (Thalassarche cauta) population trends, environmental and anthropogenic drivers, and the future for management and conservation. PhD thesis. University of Tasmania, Hobart, Tas.
Alderman R (2018) Shy albatross in Australia: population and conservation assessment report for the 2017/18 season. Marine Conservation Program, Department of Primary Industries, Water and Environment. Hobart, Tas.
Alderman R & Hobday AJ (2017) Developing a climate adaption strategy for vulnerable seabirds based on prioritisation of intervention options. Deep-Sea Research II 140, 290-297.
Alderman R, Gales R, Tuck GN & Lebreton JD (2011) Global population status of shy albatross and an assessment of colony-specific trends and drivers. Wildlife Research 38, 672-686.
Alderman R, Tuck GN, Castillo-Jordán C, Haddon M, & Punt AE (2019) Macquarie Island’s northern giant petrels and the impacts of pest eradication. Ecological Modelling 393, 66-75.
Alfaro-Shigueto J, Mangel JC, Valenzuela K & Arias-Schreiber M (2016) The intentional harvest of Waved albatrosses Phoebastria irrorata by small-scale offshore fishermen from Salaverry Port, Peru. Pan-American Journal of Aquatic Sciences 11, 70-77.
Allsop M, Walters A, Santillo D & Johnston P (2006) Plastic Debris in the World's Oceans. Greenpeace International, Amsterdam, Netherlands.
Altizer S, Ostfeld RS, Johnson PTJ, Kutz S, & Harvell CD (2013) Climate change and infectious diseases: from evidence to a predictive framework. Science 341, 514-519.
Anderson ORJ, Small CJ, Croxall JP, Dunn EK, Sullivan BJ, Yates O & Black A (2011) Global seabird bycatch in longline fisheries. Endangered Species Research 14, 91-106.
Andrady AL (2011) Microplastics in the marine environment. Marine Pollution Bulletin 62, 1596-1605.
Arata J & Xavier JC (2003) The diet of Black-browed albatrosses at the Diego Ramírez Islands, Chile. Polar Biology 26, 638-647.
ATCM (Antarctic Treaty Consultative Meeting) (2013) Management Plan for Antarctic Specially Protected Area No 160, Frazier Islands, Windmill Islands, Wilkes Land, East Antarctica. ATCM Measure 14 (2013). Available on the Internet at: https://documents.ats.aq/recatt/Att527 e.pdf.
ATCM (2015) Management Plan for Antarctic Specially Protected Area No. 102, Rookery Islands, Home Bay, Mac.Robertson Land. ATCM Measure 2 (2015). Available on the Internet at: https://documents.ats.aq/recatt/att563_e.pdf.
ATCM (2016) Management Plan for Antarctic Specially Protected Area No. 167, Hawker Island, Princess Elizabeth Land. ACTM Measure 8 (2016). Available on the Internet at: https://documents.ats.aq/recatt/att595_e.pdf.
Auman HJ, Ludwig JP, Summer CL, Verbrugge DA, Froese KL, Colborn T & Geisy JP (1997) PCBs, DDE, DDT and TCDD-EQ in two species of albatross on Sand Island, Midway Atoll, North Pacific Ocean. Environmental Toxicology and Chemistry 16, 498—504.
Awkerman JA, Huyvaert KP, Mangel J, Shigueto JA & Anderson DJ (2006) Incidental and intentional catch threatens Galápagos Waved albatross. Biological Conservation 133, 483-489.
Baird SF, Brewer TM & Ridgway R (1884) The water birds of North America, Vol 2. Little Brown And Company, Boston.
Baker AJ & Coleman JD (1977) The breeding cycle of the Westland Black Petrel. Notornis 24: 211-231.
Baker B & Hamilton S (2016) Impacts of purse-seine fishing on seabirds and approaches to mitigate bycatch. Agreement for the Conservation of Albatrosses and Petrels, Seventh Meeting of the Seabird Bycatch Working Group, Agreement for the Conservation of Albatrosses and Petrels (ACAP). 2-4 May 2016, La Serena, Chile, SBWG7 Inf 11.
Baker GB, Double MC, Gales R, Tuck GN, Abbott CL, Ryan PG, Petersen SL, Robertson CJR & Alderman R (2007) A global assessment of the impact of fisheries-related mortality on Shy and White-capped albatrosses: conservation implications. Biological Conservation 137, 319-333.
Baker G B, Gales R, Hamilton S & Wilkinson V (2002) Albatrosses and petrels in Australia: a review of their conservation and management. Emu 102, 71-97.
Baker JM (1983) Impact of oil pollution on living resources. Commission on Ecology Papers 4, 1-48.
Barbosa A & Palacios MJ (2009) Health of Antarctic birds: a review of their parasites, pathogens and diseases. Polar Biology 32, 1095-1115.
Barbraud C & Weimerskirch H (2006) Antarctic birds breed later in response to climate change. Proceedings of the National Academy of Sciences 103, 6248-6251.
Barbraud C, Delord K, Marteau C & Weimerskirch H (2009) Estimates of population size of White-chinned Petrels and Grey Petrels at Kerguelen Islands and sensitivity to fisheries. Animal Conservation 12, 258-265.
Barbraud C, Rivalan P, Inchausti P, Nevoux M, Rolland V & Weimerskirch H (2011) Contrasted demographic responses facing future climate change in Southern Ocean seabirds. Journal of Animal Ecology 80, 89-100.
Barnes DK, Galgani F, Thompson RC & Barlaz M (2009) Accumulation and fragmentation of plastic debris in global environments. Philosophical Transactions of the Royal Society B: Biological Sciences 364, 1985-1998.
Bartle JA (1990) Sexual segregation of foraging zones in procellariiform birds: implications of accidental capture on commercial fishery long-lines of grey petrels (Procellaria cinerea). Notornis 37, 146-150.
Barton D (1979) Albatrosses in the western Tasman Sea. Emu 79, 31-35.
Bartumeus F, Giuggioli L, Louzao M, Bretagnolle V, Oro D & Levin SA (2010) Fishery discards impact on seabird movement patterns at regional scales. Current Biology 20, 215-222.
Baum J & Worm B (2009) Cascading top-down effects of changing oceanic predator abundances. Journal of Animal Ecology 78, 699-714.
Beaugrand G (2014) Pelagic ecosystems and climate change, in B Freedman (ed) Global Environmental Change. Springer, Dordrecht, Netherlands. pp. 141-150.
Becker PH, González-Solís J, Behrends B & Croxall J (2002) Feather mercury levels in seabirds at South Georgia: influence of trophic position, sex and age. Marine Ecology Progress Series 243, 261-269.
Becker PH, Goutner V, Ryan PG & González-Solís J (2016) Feather mercury concentrations in Southern Ocean seabirds: variation by species, site and time. Environmental Pollution 216, 53-263.
Bell EA, Bell BD, Sim JL & Imber MJ (2013) Notes on the distribution, behaviour and status of Grey Petrel (Procellaria cinerea) on Antipodes Island, New Zealand. Notornis 60, 269-278.
Bell EA, Sim JL & Scofield P (2011) Population parameters and distribution of the black petrels (Procellaria parkinsoni) on Great Barrier Island (Aotea Island), 2008/09. Published client report funded by Conservation Services Levy. Department of Conservation, Wellington.
Berruti A (1979) Breeding biology of the Sooty Albatrosses Phoebetria fusca and Phoebetria palpebrata. Emu 79, 161-175.
Bird J, Fuller RA & Shaw JD (in review a) Differing ecological responses of seabirds to invasive species eradication. bioRxiv doi: https://doi.org/10.1101/2021.04.07.438878.
Bird J, Fuller RA, Terauds A, McInnes JC, Pascoe PP, Travers TD, Pascoe PP, Travers TD, Alderman R & Shaw JD (in review b). Detecting seabird responses to invasive species eradication. bioRxiv doi: https://doi.org/10.1101/2021.04.07.438876.
Bird JP, Martin R, Akcakaya HR, Gilroy J, Burfield IJ, Garnett ST, Symes A, Taylor J, Sekercioglu CH & Butchart SHM (2020) Generation lengths of the world's birds and their implications for extinction risk. Conservation Biology 34, 1252-1261.
BirdLife International (2004) Tracking ocean wanderers: the global distribution of albatrosses and petrels. Results from the Global Procellariiform Tracking Workshop, 1-5 September 2003, Gordon's Bay, South Africa. BirdLife International, Cambridge.
BirdLife International (2017) Macronectes halli. The IUCN Red List of Threatened Species Available on the Internet at: http://dx.doi.org/10.2305/IUCN.UK.2017-1.RLTS.T22697859A112357183.en.
BirdLife International (2018a) Diomedea amsterdamensis. The IUCN Red List of Threatened Species 2018. Available on the Internet at: https://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22698310A132397831.en.
BirdLife International (2018b) Diomedea antipodensis. The IUCN Red List of Threatened Species 2018. Available on the Internet at: http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22728318A132656045.en.
BirdLife International (2018c) Diomedea dabbenena. The IUCN Red List of Threatened Species 2018. Available on the Internet at: http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22728364A132657527.en.
BirdLife International (2018d) Diomedea epomophora. The IUCN Red List of Threatened Species 2018. Available on the Internet at: http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22698314A132641187.en.
BirdLife International (2018e) Diomedea exulans. The IUCN Red List of Threatened Species 2018. Available on the Internet at: http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22698305A132640680.en.
BirdLife International (2018f) Diomedea sanfordi. The IUCN Red List of Threatened Species 2018. Available on the Internet at: http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22728323A132656392.en.
BirdLife International (2018g) Macronectes giganteus. The IUCN Red List of Threatened Species 2018. Available on the Internet at: http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22697852A132608499.en.
BirdLife International (2018h) Phoebetria fusca. The IUCN Red List of Threatened Species 2018. Available on the Internet at: http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22698431A132645596.en.
BirdLife International (2018i) Phoebetria palpebrata. The IUCN Red List of Threatened Species 2018. Available on the Internet at: http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22698448A132647449.en.
BirdLife International (2018j) Procellaria aequinoctialis. The IUCN Red List of Threatened Species 2018. Available on the Internet at: http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22698140A132628887.en.
BirdLife International (2018k) Procellaria cinerea. The IUCN Red List of Threatened Species 2018. Available on the Internet at: http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22698159A132630237.en.
BirdLife International (2018l) Procellaria parkinsoni. The IUCN Red List of Threatened Species 2018. Available on the Internet at: http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22698150A132629374.en.
BirdLife International (2018m) Procellaria westlandica. The IUCN Red List of Threatened Species 2018. Available on the Internet at: http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22698155A132629809.en.
BirdLife International (2018n) Thalassarche bulleri. The IUCN Red List of Threatened Species 2018. Available on the Internet at: http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22728328A132656798.en.
BirdLife International (2018o) Thalassarche carteri. The IUCN Red List of Threatened Species 2018. Available on the Internet at: http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22728372A132657962.en.
BirdLife International (2018p) Thalassarche cauta. The IUCN Red List of Threatened Species 2018. Available on the Internet at: http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22729604A132660845.en.
BirdLife International (2018q) Thalassarche chlororhynchos. The IUCN Red List of Threatened Species 2018. Available on the Internet at: http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22698425A132645225.en.
BirdLife International (2018r) Thalassarche chrysostoma. The IUCN Red List of Threatened Species 2018. Available on the Internet at: http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22698398A132644834.en.
BirdLife International (2018s) Thalassarche eremita. The IUCN Red List of Threatened Species 2018. Available on the Internet at: http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22698393A132644476.en.
BirdLife International (2018t) Thalassarche impavida. The IUCN Red List of Threatened Species 2018. Available on the Internet at: http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22728349A132657209.en.
BirdLife International (2018u) Thalassarche melanophris. The IUCN Red List of Threatened Species 2018. Available on the Internet at: http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22698375A132643647.en.
BirdLife International (2018v) Thalassarche salvini. The IUCN Red List of Threatened Species 2018. Available on the Internet at: http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22698388A132644161.en.
BirdLife International (2018w) Thalassarche steadi. The IUCN Red List of Threatened Species 2018. Available on the Internet at: http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22729609A132661314.en.
BirdLife International (South Africa) (2019) Report on the final global seabird bycatch assessment workshop, 25 February-1 March 2019, Skukuza, South Africa.
Blaber SJM, Milton DA, Farmer MJ & Smith GC (1998) Seabird breeding populations on the far northern Great Barrier Reef, Australia: trends and influences. Emu 98, 44-57.
Black A (2005) Light induced seabird mortality on vessels operating in the Southern Ocean: incidents and mitigation measures. Antarctic Science 17, 67-68.
Blakers M, Davies SJJF & Reilly PN (1984) The Atlas of Australian Birds. RAOU, Melbourne University Press, Melbourne.
Boersma PD, Davies EM & Reid WV (1988) Weathered crude oil effects on chicks of Fork-tailed Storm-petrels (Oceanodroma furcata). Archives of Environmental Contamination and Toxicology 17, 527-531.
Bourne WRP & Warham J (1966) Geographical variation in the giant petrels of the genus Macronectes. Ardea 54, 45-67.
Bried J & Jouventin P (1999) Influence of breeding success on fidelity in long-lived birds: an experimental study. Journal of Avian Biology 30, 392-398.
Brooke M (2004) Albatrosses and petrels across the world. Oxford University Press, Oxford.
Brooke M, Chambers GK, Double MC, Ryan PC & Tasker ML (2007) Report by the Taxonomy Working Group to the third meeting of the Advisory Committee. Agreement on the Conservation of Albatrosses and Petrels. Third Meeting of the Advisory Committee, 19-22 June 2007, Valdivia, Chile, AC3 Doc 12.
Brothers N (1979a) Mewstone. Corella 3, 68-69.
Brothers N (1979b) Pedra Branca. Corella 3, 58-60.
Brothers N (1991) Albatross mortality and associated bait loss in the Japanese longline fishery in the Southern Ocean. Biological Conservation 55, 255-268.
Brothers N & Ledingham R (2008) The avifauna of Bishop and Clerk Islets and its relationship to nearby Macquarie Island. Papers and Proceedings of the Royal Society of Tasmania 142, 117-121.
Brothers NP, Cooper J & Løkkeborg S (1999) The Incidental Catch of Seabirds by Longline Fisheries: Worldwide Review and Technical Guidelines for Mitigation. FAO Fisheries Circular 937. FAO, Rome.
Brothers N, Duckworth AR, Safina C & Gillman EL (2010) Seabird bycatch in pelagic longline fisheries is grossly underestimated when only using haul data. PLoS One 5: e12491.
Brothers N, Gales R, Hedd A & Robertson G (1998) Foraging movements of the shy albatross Diomedea cauta breeding in Australia — implications for interactions with longline fisheries. Ibis 140, 446-457.
Brothers N, Pemberton D, Pryor H & Halley V (2001) Tasmania's offshore islands: seabirds and other natural features. Tasmanian Museum and Art Gallery, Hobart, Tas.
Brothers NP, Reid TA & Gales RP (1997) At-sea distribution of shy albatrosses Diomedea cauta cauta derived from records of band recoveries and colour-marked birds. Emu 97, 231-239.
Brown CJ, O’Connor MI, Poloczanska ES, Schoeman DS, Buckley LB, Burrows MT, Duarte CM, Halpern BS, Pandolfi JM, Parmesan C & Richardson AJ (2016) Ecological and methodological drivers of species’ distribution and phenology responses to climate change. Global Change Biology 22, 1548-1560.
Bruderer B, Peter D & Steuri T (1999) Behaviour of migrating birds exposed to X-band radar and a bright light beam. Journal of Experimental Biology 202, 1015-1022.
Bull L (2007) Reducing seabird bycatch in longline, trawl and gillnet fisheries. Fish and Fisheries 8, 31-56.
Bunce A, Norman FI, Brothers N & Gales R (2002) Long-term trends in the Australasian Gannet (Morus serrator) population in Australia: the effect of climate change and commercial fisheries. Marine Biology 141, 263-269.
Burger AE & Fry DM (1993) Effects of oil pollution on seabirds in the northeast Pacific, in K Vermeer, KT Briggs, KH Morgan & D Siegel-Causey (eds), The status, ecology, and conservation of marine birds of the North Pacific. Canadian Wildlife Service Special Publication, Ottawa. pp. 254-263.
Burger J & Gochfeld M (2002) Effects of chemicals and pollution on seabirds, in EA Schreiber & J Burger (eds), Biology of marine birds. CRC Press, New York. pp. 485-525.
Carrick R & Ingham SE (1970) In MW Holdgate (ed), Ecology and population dynamics of Antarctic sea birds, Antarctic Ecology Vol I. Academic Press, London. pp. 20-45.
Chambers LE, Dann P, Cannell B & Woehler EJ (2014) Climate as a driver of phenological change in southern seabirds. International Journal of Biometeorology 58, 603-612.
Chambers LE, Devney CA, Congdon BC, Dunlop N, Woehler EJ & Dann P (2011) Observed and predicted effects of climate on Australian seabirds. Emu 111, 235-251.
Chastel O (1995) Influence of reproductive success on breeding frequency in 4 southern petrels. Ibis 137, 360-363.
Cherel Y & Klages N (1998) A review of the food of Albatrosses, in G Robertson & R Gales (eds), Albatross biology and conservation. Beatty & Sons, Chipping Norton, Surrey. pp. 113-136.
Cherel Y, Weimerskirch H & Trouvé C (2002) Dietary evidence for spatial foraging segregation in sympatric albatrosses (Diomedea spp.) rearing chicks at Îsles Nuageuses, Kerguelen. Marine Biology 141, 1117-1129.
Childerhouse S, Robertson C, Hockly W & Gibbs N (2003) Royal Albatross (Diomedea epomophora) on Enderby Island, Auckland Islands. DOC Science Internal Series 144. Department of Conservation. Wellington.
Chown SL, Greenslade P & Marshall DJ (2005) Terrestrial invertebrates of Heard Island, in K Green & EJ Woehler (eds), Heard Island: Southern Ocean sentinel. Beatty & Sons, Chipping Norton, Surrey.
Cleeland JB, Alderman R, Bindoff A, Lea MA, McMahon CR, Phillips RA, Raymond B, Sumner MD, Terauds A, Wotherspoon SJ & Hindell MA (2019) Factors influencing habitat use of sympatric albatrosses from Macquarie Island, Australia. Marine Ecology Progress Series 606, 221-237.
Coe JM & Rogers DB (eds) (1997) Impacts of marine debris: Entanglement of marine life in marine debris including a comprehensive list of species with entanglement and ingestion records. Springer Series on Environmental Management. Springer-Verlag, New York.
Cole M, Lindeque P, Fileman E, Halsband C, Goodhead R, Moger J & Galloway TS (2013) Microplastic ingestion by zooplankton. Environmental Science and Technology 47, 6646-6655.
Collet J & Weimerskirch H (2020) Albatrosses can memorize locations of predictable fishing boats but favour natural foraging. Proceedings of the Royal Society B. Biological Sciences 287, 20200958.
Collet J, Patrick SC & Weimerskirch H (2017) A comparative analysis of the behavioural response to fishing boats in two albatross species. Behavioural Ecology 28, 1337-1347.
Commonwealth of Australia (2014) Heard Island and McDonald Islands Marine Reserve Management Plan 2014-2024, Department of the Environment, Canberra. Available on the Internet at: http://heardisland.antarctica.gov.au/protection-and-management/management-plan/download-the-heard-island-and-mcdonald-islands-marine-reserve-management-plan.
Commonwealth of Australia (2018a) Threat Abatement Plan for the impacts of marine debris on the vertebrate wildlife of Australia's coasts and oceans. Department of the Environment and Energy, Canberra. Available on the Internet at: http://www.environment.gov.au/approved-taps.
Commonwealth of Australia (2018b) Threat Abatement Plan for the incidental catch (or bycatch) of seabirds during oceanic longline fishing operations (2018). Department of the Environment and Energy, Canberra. Available on the Internet at: http://www.environment.gov.au/approved-taps.
Commonwealth of Australia (2019a) Australia's Strategy for Nature 2019-2030. Department of the Environment and Energy, Canberra. Available on the Internet at: https://www.australiasnaturehub.gov.au/national-strategy.
Commonwealth of Australia (2019b) National light pollution guidelines for wildlife including marine turtles, seabirds and migratory shorebirds, Department of the Environment and Energy, Canberra. Available on the Internet at: https://www.awe.gov.au/environment/biodiversity/publications/national-light-pollution-guidelines-wildlife.
Condie HM, Grant A & Catchpole TL (2014) Incentivising selective fishing under a policy to ban discards: lessons from European and global fisheries. Marine Policy 45, 287-292.
Copson GR (1988) The status of Black-browed and Grey-headed albatrosses on Macquarie Island. Papers and Proceedings of the Royal Society of Tasmania 122, 137-141.
Crossin GT, Trathan P & Crawford RJM (2013) Macaroni Penguin (Eudyptes chrysolophus) and Royal Penguin (Eudyptes schlegeli), in PG Borboroglu & PD Boersma (eds), Penguins: Natural History and Conservation. University of Washington Press, Seattle. pp. 185-208.
Croxall JB, Croxall JP, Butchart SHM, Lascelles B, Stattersfield AJ, Sullivan B, Symes A & Taylor P (2012) Seabird conservation status, threats and priority actions: a global assessment. Bird Conservation International 22, 1-34.
Croxall JP & Gales R (1998) An assessment of the conservation status of albatrosses, in G Robertson & R Gales (eds), Albatross biology and conservation. Beatty & Sons, Chipping Norton, Surrey. pp. 46-65.
Croxall JP & Prince PA (1994) Dead or alive, night or day: how do albatrosses catch squid? Antarctic Science 6, 155-162.
Croxall J & Rothery P (1991) Population regulation of seabirds: implications of their demography, in C Perrins, J Lebreton & G Hirons (eds), Bird Population Studies: Relevance to Conservation and Management. Oxford University Press, New York. pp. 272-296.
Croxall JP, North AW & Prince PA (1988) Fish prey of the Wandering Albatross Diomedea exulans at South Georgia. Polar Biology 9, 9-16.
Croxall JP, Prince PA, Hunter I, McInnes SJ & Copestake PG (1984) The seabirds of the Antarctic Peninsula, islands of the Scotia Sea, and the Antarctic continent between 80°W and 20°W: their status and conservation, in JP Croxall, PGH Evans & RW Schreiber (eds), Status and Conservation of the World's Seabirds. ICBP Technical Publication 2, Cambridge. pp. 293-303.
Croxall JP, Prince PA, Rothery P & Wood AG (1998) Population changes in albatrosses at South Georgia, in G Robertson & R Gales (eds), Albatross biology and conservation. Beatty & Sons, Chipping Norton, Surrey. pp. 69-83.
Croxall JP, Rothery P, Pickering SPC & Prince PA (1990) Reproductive performance, recruitment and survival of Wandering albatrosses Diomedea exulans at Bird Island, South Georgia. Journal of Animal Ecology 59, 775-796.
Croxall JP, Silk JRD, Phillips RA, Afanasyev V & Briggs DR (2005) Global circumnavigations: tracking year-round ranges of non-breeding albatrosses. Science 307, 249-250.
Cumpston JS (1968) Macquarie Island. Australian Antarctic Division, Canberra.
Cuthbert RJ, Phillips RA & Ryan PG (2003a) Separating the Tristan Albatross and the Wandering Albatross using morphometric measurements. Waterbirds 26, 338-344.
Cuthbert R, Sommer E, Ryan P, Cooper J & Hilton G (2004) Demography and conservation of the Tristan albatross Diomedea (exulans) dabbenena. Biological Conservation 117, 471-481.
Cuthbert R, Ryan PG, Cooper J & Hilton G (2003b) Demography and population trends of the Atlantic Yellow-nosed Albatross. The Condor 105, 439-452.
Danckwerts DK, McQuaid CD, Jaeger A, McGregor GK, Dwight R, Le Corre M & Jaquemet S (2014) Biomass consumption by breeding seabirds in the western Indian Ocean: indirect interactions with fisheries and implications for management. ICES Journal of Marine Science 71, 2589-2598.
Davies D, Dilley BJ, Bond AL, Cuthbert RJ & Ryan PG (2015) Trends and tactics of mouse predation on Tristan Albatross Diomedea dabbenena chicks at Gough Island, South Atlantic Ocean. Avian Conservation and Ecology 10(1): 5.
DAWE (Department of Agriculture, Water and the Environment) (2020) Register of critical habitat. Available on the Internet at: https://www.environment.gov.au/cgi-bin/sprat/public/publicregisterofcriticalhabitat.pl.
DAWR (Department of Agriculture and Water Resources) (2018) National Plan of Action for minimising incidental catch of seabirds in Australian capture fisheries. Canberra, ACT. Available on the Internet at: https://www.awe.gov.au/agriculture-land/fisheries/environment/bycatch/seabirds#:~:text=The%20Australian%20Government%20has%20developed,impact%20of%20fishing%20on%20seabirds.
Debski I & Pierre J (2014) Seabird cryptic mortality and risk from fisheries. South Pacific regional Fisheries Management Organisation, Second Meeting of the SPRFMO Scientific Committee, 1-7 October 2014, Honolulu, Hawaii, SC-02-13.
Department of the Environment (2016) Review of national recovery plan for threatened albatrosses and giant petrels 2011-2016. Department of the Environment, Australian Antarctic Division, Hobart. Available on the Internet at: https://www.awe.gov.au/environment/biodiversity/threatened/recovery-plans/national-recovery-plan-threatened-albatrosses-and-giant-petrels-2011-2016.
DEH (Department of the Environment and Heritage) (2001) Recovery Plan for albatrosses and giant-petrels. Commonwealth of Australia, Canberra.
DEH (2002) Map of habitat critical to the survival of survival of three species of albatross Diomedea exulans (wandering albatross), Thalassarche cauta (Shy Albatross) and Thalassarche chrysostoma (Grey-headed Albatross). Available on the Internet at: http://www.environment.gov.au/node/14570.
de la Mare WK & Kerry KR (1994) Population dynamics of the Wandering Albatross (Diomedea exulans) on Macquarie Island and the effect of mortality from long-line fishing. Polar Biology 14, 231-241.
del Hoyo J & Collar NJ (2014) HBW and BirdLife International illustrated checklist of the birds of the world. Volume 1: non-passerines. Lynx Edicions, Barcelona.
Deutsch C, Ferrel A, Seibel B, Pörtner HO & Huey RB (2015) Climate change tightens a metabolic constraint on marine habitats. Science 348, 1132-1135.
Dias MP, Martin R, Pearmain EJ, Burfield IJ, Small C, Phillips RA, Yates O, Lascelles B, Borboroglu PG & Croxall JP (2019) Threats to seabirds: a global assessment. Biological Conservation 237, 525-537.
Dierschke V, Furness RW & Garthe S (2016) Seabirds and offshore wind farms in European waters: Avoidance and attraction. Biological Conservation 202, 59-68.
Dietrich KS, Cornish VR, Rivera KR & Conant TA (2004) Best practices for the collection of longline data to facilitate research and analysis to reduce by-catch of protected species. Report of a workshop held at the International Fisheries Observer Conference, 8 November 2004, Sydney, NSW.
Dilley BJ, Davies D, Connan M, Cooper J, de Villiers M, Swart L, Vandenabeele S, Ropert-Coudert Y & Ryan PG (2013) Giant petrels as predators of albatross chicks. Polar Biology 36, 761-766.
Dixon CC (1933) Some observations on the albatrosses and other birds of the Southern Ocean. Transactions of the Royal Canadian Institute 19, 117-119.
Double M (2006) Report by the Taxonomy Working Group to the second meeting of the Advisory Committee. Agreement on the Conservation of Albatrosses and Petrels. Second Meeting of the Advisory Committee, 5-8 June 2006, Brasilia, Brazil, AC2 Doc 11.
Downes M (2002) First visitors to Heard Island. ANARE Research Notes, Australian Antarctic Division, Department of the Environment and Heritage.
Downes MC, Ealey EHM, Gwynne AM & Young PS (1959) The birds of Heard Island. Australian National Antarctic Research Expedition Reports (Series B).
DPIPWE (Department of Primary Industries, Parks, Water and Environment) (2016) Tasmanian Wilderness World Heritage Area Management Plan 2016. Department of Primary Industries, Parks, Water and Environment, Hobart, Tas.
DPIPWE (2021a) Population and conservation status of Macquarie Island albatrosses and giant petrels: Report for the 2020/21 season. Marine Conservation Program, Department of Primary Industries, Parks, Water and Environment, Hobart, Tas.
DPIPWE (2021b) Shy Albatross in Australia: Population and conservation assessment report for the 2020/21 season. Marine Conservation Program, Department of Primary Industries, Parks, Water and Environment, Hobart, Tas.
DSEWPC (Department of Sustainability, Environment, Water, Population and Communities) (2011a) Background paper: population status and threats to albatrosses and giant petrels listed as threatened under the Environment Protection and Biodiversity Conservation Act 1999. Commonwealth of Australia, Hobart, Tas. Available on the Internet at: https://www.environment.gov.au/resource/background-paper-population-status-and-threats-albatrosses-and-giant-petrels-listed.
DSEWPC (2011b) National Recovery Plan for threatened albatrosses and giant petrels: 2011–2016. Commonwealth of Australia, Hobart, Tas. Available on the Internet at: https://www.environment.gov.au/biodiversity/threatened/recovery-plans/national-recovery-plan-threatened-albatrosses-and-giant-petrels-2011-2016.
Eisenbud R (1985) Problems and prospects for the pelagic driftnet. Boston College Environmental Affairs Legal Review 12, 473-490.
Elliot G & Walker K (2020) Antipodean wandering albatross census and population study 2020. Prepared by the Department of Conservation, Wellington, New Zealand.
Elliot GP, Walker KJ, Nicholls DG & Murray MD (1995) Foraging patterns of Wandering Albatrosses from the Auckland Islands, in Australian Nature Conservation Agency, Abstracts from the First International Conference on the Biology and Conservation of Albatrosses. 28 August to 1 September 1995, Hobart Tas.
Falla RA (1933) Notes on New Zealand petrels with descriptions of new forms and some new records. Records of the Auckland Institute and Museum 1, 173-180.
Falla RA (1946) An undescribed form of the black petrel. Records of the Canterbury Museum 5, 111-113.
FAO (Food and Agriculture Organization of the United Nations) (1995) Code of Conduct for responsible fisheries. Food and Agriculture Organization of the United Nations, Rome. Available on the Internet at: http://www.fao.org/fishery/code/en.
FAO (1999) International Plan of Action for reducing incidental catch of seabirds in longline fisheries. Food and Agriculture Organization of the United Nations, Rome. Available on the Internet at: http://www.fao.org/fishery/ipoa-seabirds/en.
FAO (2009) Fishing Operations 2. Best Practices to Reduce Incidental Catch of Seabirds in Capture Fisheries. Food and Agriculture Organization of the United Nations, Rome. Available on the Internet at: http://www.fao.org/in-action/globefish/publications/details-publication/en/c/346107/.
FAO (2016) The state of world fisheries and aquaculture in 2016: Contributing to Food Security and Nutrition for All. Food and Agriculture Organization of the United Nations, Rome.
Favero M & Seco Pon JP (2014) Challenges in seabird by-catch mitigation. Animal Conservation 17, 532-533.
Fondo EN, Chaloupka M, Heymans JJ & Skilleter GA (2015) Banning fisheries discards abruptly has a negative impact on the population dynamics of charismatic marine megafauna. PLoS One 10: e0144543.
Forster JR (1785) Mémoire sur les Albatros. Memoires de Mathematique et de Physique. Paris (Acad. Sci.) 10, 563-572, pls. xiii-xv.
Francis RICC (2012) Fisheries risk to the population viability of White-capped Albatross Thalassarche steadi. New Zealand Aquatic Environment and Biodiversity Report No. 104. Ministry for Primary Industries, Wellington, New Zealand.
Francis, RIC & Sagar PM (2012) Modelling the effect of fishing on Southern Buller’s Albatross using a 60-year dataset. New Zealand Journal of Zoology 39, 3-17.
FRDC (Fisheries Research and Development Corporation) (2020) Improving the effectiveness, efficiency and safety of mitigation tools for protected species interactions in the Eastern Tuna and Billfish Fishery. Project No. 2020-041. Available on the Internet at: https://www.frdc.com.au/project/2020-041.
Fry DM & Lowenstine LJ (1985) Pathology of common murres and Cassin's auklets exposed to oil. Archives of Environmental Contamination and Toxicology 14, 725-737.
Fry DM, Fefer SI & Silo L (1987) Ingestion of plastic debris by Laysan albatrosses and Wedge-tailed shearwaters in the Hawaiian Islands. Marine Pollution Bulletin 18, 339-343.
Gales R (1998) Albatross populations: status and threats, in G Robertson & R Gales (eds), Albatross biology and conservation. Beatty & Sons, Chipping Norton, Surrey. pp. 20-45.
Gales R & Brothers N (1996) Status and Conservation of Albatrosses on Macquarie Island. Australian Nature Conservation Agency Report, SCA10636.
Gall SC & Thompson RC (2015) The impact of debris on marine life. Marine Pollution Bulletin 92, 170-179.
Garnett ST & Baker GB (Eds) (2021) The Action Plan for Australian Birds 2020. CSIRO Publishing, Melbourne, Vic.
Gauthier G, Milot E & Weimerskirch H (2010) Small-scale dispersal and survival in a long-lived seabird, the wandering albatross. Journal of Animal Ecology 79, 879-887.
GESAMP (Group of Experts on the Scientific Aspects of Marine Environmental Protection) (1993) Impact of oil and related chemicals on the marine environment. Joint Group of Experts on the Scientific Aspects of Marine Pollution. IMO Reports and Studies No. 50, London.
Gianuca D, Bugoni L, Jiménez S, Daudt NW, Miller P, Canani G, Silva-Costa A, Faria FA, Bastida J, Seco Pon JP, Yates O, Serafina PP & Bond AL (2020) Intentional killing and extensive aggressive handling of albatrosses and petrels at sea in the southwestern Atlantic Ocean. Biological Conservation 252: 108817.
Gmelin JF (1789) Systema naturae per regna tria naturae, secundum classes, ordines, genera, species cum characteribus, differentiis, synonymis, locis. Edito decima tertia, aucta reformata, cura JF Gmelin. Lepizig.
Goldsworthy SD, Giese M, Gales RP, Brothers N & Hamill J (2000) Effects of the Iron Baron oil spill on little penguins (Eudyptula minor). II. Post-release survival of rehabilitated oiled birds. Wildlife Research 27, 573-582.
González-Solis J (2004) Sexual size dimorphism in Northern Giant Petrels: ecological correlates and scaling. Oikos 105, 247-254.
González Zevallos D & Yorio P (2006) Seabird use of discards and incidental captures at the Argentine hake trawl fishery in Golfo San Jorge, Argentina. Marine Ecology Progress Series 316, 175-183.
Gorta SBZ, Smith JA, Everett JD, Kingsford RT, Cornwell WK, Suthers IM, Epstein H, McGovern R, McLachlan G, Roderick M, Smith L, Williams D & Callaghan CT (2019) Pelagic citizen science data reveal declines of seabirds off south-eastern Australia. Biological Conservation 235, 226-235.
Gould J (1841) Descriptions of new birds from Australia. Proceedings of the Zoological Society of London 8, 177-178.
Goutte A, Barbraud C, Meillère A, Carravieri A, Bustamante P, Budzinski H, Delord K, Cherel Y, Weimerskirch H & Chastel O (2014) Demographic consequences of heavy metals and persistent organic pollutants in a vulnerable long-lived bird, the Wandering Albatross. Proceedings of the Royal Society B: Biological Sciences 281: 20133313.
Gray GR (1862) A list of the birds of New Zealand and adjacent islands. Ibis 4, 214-252.
Green K, Kerry K, Disney T & Clarke M (1998) Dietary studies of Light-mantled Sooty Albatrosses Phoebetria palpebrata from Macquarie and Heard Islands. Marine Ornithology 26, 19-26.
Green RH (1974) Albatross Island, 1973. Records of the Queen Victoria Museum 51, 1-17.
Gremillet D, Peron C, Provost P & Lescroel A (2015) Adult and juvenile European seabirds at risk from marine plundering off West Africa. Biological Conservation 182, 143-147.
Guilford T, Padget O, Maurice L & Catry P (2022) Unexpectedly deep diving in an albatross. Current Biology doi: https://doi.org/10.1016/j.cub.2021.11.036
Hall AJ (1987) The Breeding Biology of the White-Chinned Petrel Procellaria aequinoctialis at South Georgia. Journal of Zoology 212, 605-617.
Harper PC (1987) Feeding behaviour and other notes on 20 species of Procellariiformes at sea. Notornis 34, 169-192.
Harper PC, Croxall JP & Cooper J (1985) A guide to foraging methods used by marine birds in Antarctic and sub-Antarctic seas. BIOMASS handbook, No. 24, University of California, Davis, California.
Headland RK (1993) Chronological List of Antarctic Expeditions and Related Historical Events. Cambridge University Press, Cambridge, UK.
Hedd A & Gales R (2001) The diet of shy albatross (Thalassarche cauta) at Albatross Island, Tasmania. Journal of the Zoological Society of London, 253, 69-90.
Hedd A & Gales R (2005) Breeding and overwintering ecology for shy albatross in southern Australia: Year round patterns of colony attendance and foraging trip durations. The Condor 107, 375-387.
Hedd A, Gales R & Brothers N (2001) Foraging strategies of Shy Albatross Thalassarche cauta at Albatross Island Tasmania, Australia. Marine Ecology Progress Series 224, 267-282.
Hedd A, Gales R, Brothers N & Robertson G (1997) Diving behaviour of the Shy albatross Diomedea cauta in Tasmania: initial findings and dive recorder assessment. Ibis 139, 452-460.
Higginbottom K (2004) Wildlife tourism: impacts, management and planning. Cooperative Research Centre for Sustainable Tourism, Common Ground Publishing, Altona.
Hilsenberg (1822) Diomedea fusca. Froriep's Notizen Vol III, No 5 (49), p.74.
Hindell MA & Burton HR (1988) The history of the Elephant Seal industry at Macquarie Island and an estimate of the pre-sealing numbers. Papers and Proceedings of the Royal Society of Tasmania 122, 159-176.
Hindell M, Brothers N & Gales R (1999) Mercury and cadmium concentrations in the tissues of three species of southern albatrosses. Polar Biology 22, 102-108.
Huin N & Croxall JP (1996) Fishing Gear, oil and marine debris associated with seabirds at Bird Island South Georgia, during 1993/94. Marine Ornithology 24, 190-194.
Hunter S (1983) The food and feeding ecology of the giant petrels Macronectes halli and M. giganteus at South Georgia. Journal of Zoology 200, 521-538.
Imber MJ (1987) Breeding ecology and the conservation of the black petrel (Procellaria parkinsoni). Notornis 34, 19-39.
Imber MJ, McFadden I, Bell EA & Scofield RP (2003) Post-fledging migration, age of first return and recruitment, and results of inter-colony translocation of black petrels (Procellaria parkinsoni). Notornis 50, 183-190.
Irons DB, Kendall SJ, Erickson WP & McDonald LL (2000) Nine years after the Exxon Valdez oil spill: effects on marine bird populations in Prince William Sound, Alaska. Condor 102, 723-737.
IUCN (International Union for Conservation of Nature) (2016) A Global Standard for the Identification of Key Biodiversity Areas, Version 1.0. First edition. Gland, Switzerland: IUCN.
IUCN (2021) IUCN Red List of Threatened Species, Version 2021-3. Available on the Internet at: iucnredlist.org.
Jackson R (1958) The Westland Petrel. Notornis 7,230-233.
Jambeck J R, Geyer R, Wilcox C, Siegler T R, Perryman M, Andrady A & Law, K L (2015) Plastic waste inputs from land into the ocean. Science 347, 768-771.
Jiménez S, Marquez A, Abreu M, Forselledo R, Pereira A & Domingo A (2015) Molecular analysis suggests the occurrence of Shy Albatross in the south-western Atlantic Ocean. Emu 115, 58-62.
Johnstone GW (1980) Australian islands in the Southern Ocean. Bird Observer 586, 85-87.
Johnstone GW (1982) Zoology, in Expedition to the Australian Territory of Heard Island and McDonald Islands 1980. Technical Report 31. Department of National Development and Energy, Division of Mapping, Canberra, ACT. pp. 33-39.
Johnstone GW, Milledge D & Dorward DF (1975) The white-capped albatross of Albatross Island: numbers and breeding behaviour. Emu 75, 1-11.
Jones E (1980) A survey of burrow-nesting petrels at Macquarie Island based on remains left by predators. Notornis 27, 11-20.
Jouventin P & Weimerskirch H (1988) Demographic strategies of southern albatrosses. Acta XIX Congressus Internationalis Ornithologici 857-865.
Jouventin P & Weimerskirch H (1990) Satellite tracking of wandering albatrosses. Nature 343, 746-748.
Jouventin P & Weimerskirch H (1991) Changes in the population size and demography of southern seabirds: management implications, in CM Perrins, JD Lebreton & GM Hirons (eds), Bird population studies: their relevance to conservation and management. Oxford University Press, Oxford. pp. 297-316.
Jouventin P, Martinez J & Roux JP (1989) Breeding biology and current status of the Amsterdam Island Albatross. Ibis 131, 171-189.
Jouventin P, Mougin JL, Stahl JC & Weimerskirch H (1985) Comparative breeding biology of the burrowing petrels at the Crozet Islands. Notornis 32, 157-220.
Jouventin P, Roux JP, Stahl JC & Weimerskirch H (1983) La biologie et la frequence de reproduction de Diomedea chlororhynchos. Le Gerfaut 73, 161-171.
Kärrman A, Schönlau C & Engwall M (2016) Exposure and effects of microplastics on wildlife: a review of existing data. MTM Research Centre, Örebro University, Sweden.
KBA Standards and Appeals Committee (2019) Guidelines for using a Global Standard for the Identification of Key Biodiversity Areas. Version 1.0. Prepared by the KBA Standards and Appeals Committee of the IUCN Species Survival Commission and IUCN World Commission on Protected Areas. IUCN, Gland, Switzerland. viii + 148pp.
Keage PL & Johnstone GW (1982) Heard Island and the McDonald Islands. Australian Heritage Commission Newsletter 5, 4-5.
Kirkwood RJ, Woehler EJ & Burton HR (1989) Heard Island 1987/88 Australian National Antarctic Research Expedition Report. Unpublished report to Department of National Development. Melbourne.
Kirkwood R, Downes D, Moore G, Copley P & Tideman J (1995) The decline of Southern Giant-Petrels at Heard Island, in Abstracts from First International Conference on the Biology and Conservation of Albatrosses, Hobart (Tasmania), 28 August to 1 September 1995. Australian Nature Conservation Agency, Hobart, Tas. pp. 48.
Koopman M, Boag S, Tuck GN, Hudson R, Knuckey I & Alderman R (2018) Industry-based development of effective new seabird mitigation devices in the southern Australian trawl fisheries. Endangered Species Research 36, 197-211.
Kühn SE, Rebolledo B & Franeker J (2015) Deleterious effects of litter on marine life, in M Bergmann, L Gutow & M Klages (eds), Marine anthropogenic litter. Springer International Publishing, Cham. Pp. 75-116.
Laist DW (1997) Impacts of marine debris: Entanglement of marine life in marine debris including a comprehensive list of species with entanglement and ingestion records, in JM Coe & DB Rogers (eds), Marine Debris. Springer Series on Environmental Management. Springer, New York, NY. pp. 99-139.
Lance BK, Irons DB, Kendall SJ & McDonald LL (2001) An evaluation of marine bird population trends following the Exxon Valdez oil spill, Prince William Sound, Alaska. Marine Pollution Bulletin 42, 298-309.
Lawton K, Kirkwood R, Robertson G & Raymond B (2007) Preferred foraging areas of Heard Island albatrosses during chick raising and implications for the management of incidental mortality in fisheries. Aquatic Conservation: Marine and Freshwater Ecosystems, 18, 309-320.
Le Bohec C, Gauthier-Clerc M, Gendner JP, Chatelain N & Le Maho Y (2003) Nocturnal predation of King penguins by giant petrels on the Crozet Islands. Polar Biology 26, 587-590.
Le Corre M & Jaquemet S (2005) Assessment of the seabird community of the Mozambique Channel, and its potential use as an indicator of tuna abundance. Estuarine Coastal and Shelf Science 63, 421-428.
Lesson RP (1825) Distribution géographique de quelques Oiseaux marins, observés dans le voyage autour du monde de la corvette La Coqille. Annales des Sciences Naturelles 6, 88-103.
Lewison RL, Crowder LB, Wallace BP, Moore JE, Cox T, Žydelis R, McDonald S, DiMatteo A, Dunn DC, Kot CY & Bjorkland R (2014) Global patterns of marine mammal, seabird, and sea turtle bycatch reveal taxa-specific and cumulative megafauna hotspots. Proceedings of the National Academy of Sciences 111, 5271-5276.
Linnaeus C (1758) Systema naturae per regna tria naturae, secundum classes, ordines, genera, species cum characteribus, differentiis, synonymis, locis. Laurenttii Salvii.
Lock JW, Thompson DR, Furness RW & Bartle JA (1992) Metal concentrations in seabirds of the New Zealand region. Environmental Pollution 75, 289-300.
Løkkeborg S (2011) Review and assessment of mitigation measures to reduce incidental catch of seabirds in longline and trawl fisheries. Marine Ecology Progress Series 435, 285-303.
Longcore T & Smith PA (2013) On avian mortality associated with human activities. Avian Conservation and Ecology 8(2): 1.
Ludwig JP, Summer CL, Auman HJ, Gauguer G, Bromley D, Giesy JP, Rolland R & Colborn T (1998) The roles of organochlorine contaminants and fisheries by-catch in recent population changes of Black-footed and Laysan Albatrosses in the North Pacific Ocean, in G Robertson, & R Gales (eds), Albatross biology and conservation. Beatty & Sons, Chipping Norton, Surrey. pp. 225-238.
Luke BG, Johnstone GW & Woehler EJ (1989) Organochlorine pesticides, PCBs and mercury in Antarctic and sub-Antarctic seabirds. Chemosphere 19, 2007-2021.
Lyle JM, Bell JD, Chuwen BM, Barrett N, Tracey SR & Buxton CD (2014) Assessing the impacts of gillnetting in Tasmania: implications for by-catch and biodiversity. Fisheries Research and Development Corporation, Canberra ACT, and Institute for Marine and Antarctic Studies, University of Tasmania, Hobart, Tas.
Marchant S & Higgins P (eds) (1990) Handbook of Australian, New Zealand and Antarctic birds. Vol 1 Rarities to Ducks. Oxford University Press, Melbourne, Vic.
Maree BA, Wanless RM, Fairweather TP, Sullivan BJ & Yates O (2014) Significant reductions in mortality of threatened seabirds in a South African trawl fishery. Animal Conservation 17, 520-529.
Mariano-Jelicich R, Copello Seco Pon JP & Favero M. (2014) Contribution of fishery discards to the diet of the Black-browed Albatross (Thalassarche melanophris) during the non-breeding season: an assessment through stable isotope analysis. Marine Biology 161, 119-129.
Marshall GJ (2003) Trends in the Southern Annular Mode from observations and reanalyses. Journal of Climate 16, 4134-4143.
Mathews GM (1912) Birds of Australia. Vol 2. Witherby, London.
Matthews LH (1929) The birds of South Georgia. 'Discovery' Rep. 1, 561-592.
McClelland GTW, Bond AL, Sardana A & Glass T (2016) Rapid population estimate of a surface-nesting seabird on a remote island using a low-cost unmanned aerial vehicle. Marine Ornithology 44, 215-220.
McInnes JC, Alderman R, Deagle BE, Lea M-A, Raymond B & Jarman SN (2017a) Optimised scat collection protocols for dietary DNA metabarcoding in vertebrates. Methods in Ecology and Evolution 8, 192-202.
McInnes JC, Jarman SN, Lea M-A, Raymond B, Deagle BE, Phillips RA, Catry P, Stanworth A, Weimerskirch H, Kusch A, Gras M, Cherel Y, Maschette D & Alderman R (2017b) DNA Metabarcoding as a Marine Conservation and Management Tool: A Circumpolar Examination of Fishery Discards in the Diet of Threatened Albatrosses. Frontiers in Marine Science 4: 277. doi: 10.3389/fmars.2017.00277.
McInnes JC, Tuck GN & Alderman R (2020) Using scat DNA to inform sustainable fisheries management and Ecological Risk Assessments: a Shy Albatross case study, Department of Primary Industries, Parks, Water and Environment (Tas). Hobart.
McInnes KL, O’Grady JG, Hemer M, Macadam I, Abbs DJ, White CJ, Corney SP, Grose MR, Holz GK, Gaynor SM & Bindoff NL (2012) Climate futures for Tasmania: extreme tide and sea-level events technical report. Antarctic Climate and Ecosystems Cooperative Research Centre, Hobart, Tas.
Melvin EF, Dietrich KS, Fitzgerald S & Cardoso T (2011) Reducing seabird strikes with trawl cables in the pollock catcher-processor fleet in the eastern Bering Sea. Polar Biology 34, 215-226.
Menkhorst P, Rogers D, Clarke R, Davies J, Marsack P & Franklin K (2017) The Australian Bird Guide. CSIRO Publishing, Victoria, Australia.
Miskelly CM, Sagar PM, Tennyson AJD & Scofield RP (2001) Birds of the Snares Islands, New Zealand. Notornis 48, 1-40.
Moore JL (1999) Norfolk Island Bird Notes, 1977 to 1997. Notornis 46, 354-364.
Moore PJ, Scott JJ, Joyce LJ & Peart M (1997) Southern Royal Albatross Diomedea epomophora epomophora census on Campbell Island, 4 January to 6 February 1996, and a review of population figures. Science & Research Series No. 101, Department of Conservation, Wellington, New Zealand.
Moors PJ & Atkinson IAE (1984) Predation on seabirds by introduced animals, and factors affecting its severity, in JP Croxall, PGH Evans & RW Schreiber (eds). Status and Conservation of the World's Seabirds. CBP Technical Publication 2, Cambridge. pp. 667-690.
Muirhead SJ & Furness RW (1988) Heavy metal concentrations in the tissues of seabirds from Gough Island, South Atlantic Ocean. Marine Pollution Bulletin 16, 278-283.
Murphy RC (1917) A new albatross from the west coast of South America. Bulletin of the American Museum of Natural History 37, 861-864.
Murphy RC (1930) Birds collected during the Whitney South Sea Expedition. XI. American Museum Novitates 419, 1-15.
Murphy RC (1936) Oceanic birds of South America. American Museum of Natural History, New York.
Nel DC, Ryan PG & Cooper J (2002) Population dynamics of Wandering albatrosses Diomedea exulans at sub-Antarctic Marion Island: longline fishing and environmental influences. Commission for the Conservation of Antarctic Marine Living Resources, Second Meeting of the Working Group on Fish Stock Assessment, 7-17 October 2002, CCAMLR WG-FSA-02/16.
Nicholls D, Murray D, Battam H, Robertson G, Moors P, Butcher E & Hildebrandt M (1995) Satellite tracking of the Wandering albatross Diomedea exulans around Australia and in the Indian Ocean. Emu 95, 223-230.
Nicholls DG, Murray MD, Butcher E & Moors P (1997) Weather systems determine the non-breeding distribution of Wandering albatrosses over southern oceans. Emu 97, 240-244.
Nicholls DG, Murray MD, Butcher EC & Moors PJ (2000) Time spent in exclusive economic zones of southern oceans by non-breeding Wandering albatrosses (Diomedea spp.): implications for national responsibilities for conservation. Emu 100, 318-323.
Nichols JT & Murphy RC (1914) A review of the genus Phoebetria. Auk 31, 526-534.
Nunn GB & Stanley SE (1998) Body size effects and rates of cytochrome b evolution in tube-nosed seabirds. Molecular Biology & Evolution 15, 1360-1371.
Nunn GB, Cooper J, Jouventin P, Robertson CJR & Robertson GG (1996) Evolutionary relationships among extant albatrosses (Procellariiformes: Diomedeidae) established from complete cytochrome-b gene sequences. Auk 113, 784-801.
Onley D & Scofield (2007) Albatrosses, Petrels and Shearwaters of the World. Helm Field Guides London.
Orams MB (2002) Feeding wildlife as a tourism attraction: a review of issues and impacts. Tourism Management 23, 281-293.
Oro D, Genovart M, Tavecchia G, Fowler MS & Martínez-Abraín A (2013) Ecological and evolutionary implications of food subsidies from humans. Ecology Letters 16, 1501-1514.
Packer J & Ballantyne R (2013) Encouraging reflective visitor experiences in ecotourism, in R Ballantyne & J Packer (eds), International Handbook on Ecotourism. Edward Elgar Publishing, Cheltenham, UK. pp. 169-177.
Paleczny M, Hammill E, Karpouzi V & Pauly D (2015) Population trend of the world’s monitored seabirds, 1950-2010. PLoS One 10: e0129342.
Pannekoek J & van Strien A (2006) TRIM 3.53 (TRends & Indices for Monitoring data). Statistics Netherlands, Voorburg.
Pardo D, Barbraud C, Authier M & Weimerskirch H (2013) Evidence for an age-dependent influence of environmental variations on a long-lived seabird’s life-history traits. Ecology 94, 208-220.
Parker G & Molloy J (2017) Stocktake of measures for mitigating the incidental capture of seabirds in New Zealand commercial fisheries. Agreement on the Conservation of Albatrosses and Petrels. Fifth Meeting of the Seabird Bycatch Working Group, 4-6 September 2017, Wellington, New Zealand, SBWG8 Inf 20.
Parker G, Brickle P, Crofts S, Poppert J & Wolfaardt A (2013) Research into undetected seabird mortality in a demersal trawl fishery. Agreement on the Conservation of Albatrosses and Petrels. Fifth Meeting of the Seabird Bycatch Working Group, 1-3 May 2013, La Rochelle, France, SBWG5 Doc 07.
Parks and Wildlife Service (2006) Macquarie Island Nature Reserve and World Heritage Area Management Plan. Parks and Wildlife Service, Department of Tourism, Arts and the Environment, Hobart, Tas.
Parks and Wildlife Service (2014) Evaluation report: Macquarie Island Pest Eradication Project. Department of Primary Industries, Parks, Water and Environment, Hobart Tas.
Patrick SC, Bearhop S, Bodey TW, Grecian WJ, Hamer KC, Lee J & Votier SC (2015) Individual seabirds show consistent foraging strategies in response to predictable fisheries discards. Journal of Avian Biology 46, 431-440.
Patterson DL & Hunter S (1999) Giant Petrel Macronectes spp. banding recovery analysis from the International Giant Petrel Banding Project, 1988/89. Marine Ornithology 28, 69-74.
Patterson DL, Woehler EJ, Croxall JP, Cooper J, Poncet S & Fraser WR (2008) Breeding distribution and population status of the Northern Giant Petrel (Macronectes halli) and the Southern Giant Petrel (M. giganteus). Marine Ornithology 36, 115-124.
Pauly D, Christensen V, Dalsgaard J, Froese R & Torres F (1998) Fishing down marine food webs. Science 279, 860-863.
Paz JA, Seco Pon JP, Favero M, Blanco G & Copello S (2018) Seabird interactions and bycatch in the anchovy pelagic trawl fishery operating in northern Argentina. Aquatic Conservation 28, 850-860.
Phalan B, Phillips RA, Silk JR, Afanasyev V, Fukuda A, Fox J, Catry P, Higuchi H & Croxall JP (2007) Foraging behaviour of four albatross species by night and day. Marine Ecology Progress Series 340, 271-286.
Phillips RA, Gales R, Baker GB, Double MC, Favero M, Quintana F, Tasker ML, Weimerskirch H, Uhart M & Wolfaardt A (2016) A global assessment of the conservation status, threats and priorities for albatrosses and large petrels. Biological Conservation 201, 169-183.
Phillips RA, Silk JRD & Croxall JP (2005) Foraging and provisioning strategies of the Light-mantled Sooty albatross at South Georgia: competition and co-existence with sympatric pelagic predators. Marine Ecology Progress Series 285, 259-270.
Phillips RA, Silk JRD, Phalan B, Catry P & Croxall JP (2004) Seasonal sexual segregation in two Thalassarche albatross species: competitive exclusion, reproductive role specialization or foraging niche divergence? Proceedings of the Royal Society of London B 271, 1283-1291.
Piatt JF & Ford RG (1996) How many seabirds were killed by the Exxon Valdez oil spill?, in SD Rice, RB Spies, DA Wolfe & BA Wright (eds), Proceedings of the Exxon Valdez oil spill symposium. American Fisheries Society Symposium 18, Bethesda, MD. pp. 712-719.
Pickering SPC (1989) Attendance patterns and behaviour in relation to experience and pair-bond formation in the Wandering Albatross Diomedea exulans at South Georgia. Ibis 131, 183-195.
Pierce KE, Harris RJ, Larned LS & Pokras MA (2004) Obstruction and starvation associated with plastic ingestion in a Northern Gannet Morus bassanus and a Greater Shearwater Puffinus gravis. Marine Ornithology 32, 187-189.
Pinaud D & Weimerskirch H (2007) At-sea distribution and scale-dependent foraging behaviour of petrels and albatrosses: a comparative study. Journal of Animal Ecology 76, 9-19.
Prince P, Croxall JP, Trathan PN & Wood AG (1998) The pelagic distribution of South Georgia albatrosses and relationships with fisheries, in G Robertson & R Gales (eds), Albatross biology and conservation. Beatty & Sons, Chipping Norton, Surrey. pp. 137-167.
Prince PA, Huin N & Weimerskirch H (1994) Diving depths of albatrosses. Antarctic Science 6, 353-354.
Prince PA, Rothery P, Croxall JP & Wood AG (1994) Population dynamics of Black-browed and Grey-headed albatrosses Diomedea melanophrys and D. chrysostoma at Bird Island, South Georgia. Ibis 136, 50-71.
Reichenbach L (1852) Avium systema naturale. Leipzig, Germany.
Reichenow (1898) Diomedea platei. Ornith. Monatsberichte 6, 190.
Reid TA, Hindell MA, Eades DW & Newman N (2002) Atlas of the Seabirds of Southeast Australia. Monograph 4, Birds Australia, Melbourne, Vic.
Reid T & James D (1997) The Chatham Island Albatross Diomedea (cauta) eremita in Australia. Notornis 44, 125-128.
Reisser J, Shaw J, Wilcox C, Hardesty BD, Proietti M, Thums M & Pattiaratchi C (2013) Marine plastic pollution in waters around Australia: characteristics, concentrations, and pathways. PLoS One 8: e80466.
Rexer-Huber K (2017) White-chinned Petrel distribution, abundance and connectivity: NZ populations and their global context. Report to New Zealand Department of Conservation. Parker Conservation, Dunedin.
Ridoux V (1994) The diets and dietary segregation of seabirds at the subantarctic Crozet Islands. Marine Ornithology 22, 1-192.
Risi MM, Jones CW, Osborne AM, Steinfurth A & Oppel S (2021) Southern Giant Petrels Macronectes giganteus depredating breeding Atlantic Yellow-nosed Albatrosses Thalassarche chlororhynchos on Gough Island. Polar Biology 44, 593-599.
Robertson CJR (1975) Yellow-nosed mollymawk (Diomedea chlororhynchos) recorded in the Chatham Islands. Notornis 22, 342-344.
Robertson CJR (ed) (1985) The Complete Book of New Zealand Birds. Reader’s Digest Services, Sydney.
Robertson CJR (1993) Survival and longevity of the Northern Royal Albatross Diomedea epomophora sanfordi at Taiaroa Head 1937-93. Emu 93, 269-276.
Robertson CJR (1991) Questions on the harvesting of Toroa in the Chatham Islands. Science and Research Series 35. Department of Conservation, Wellington, New Zealand.
Robertson CJR (1998) Factors influencing the breeding performance of the Northern Royal Albatross, in G Robertson & R Gales (eds), Albatross biology and conservation. Beatty & Sons, Chipping Norton, Surrey. pp. 99-104.
Robertson CJR (2002) The scientific name of the Indian Yellow-nosed Albatross Thalassarche carteri. Marine Ornithology 30, 48-49.
Robertson CJ & Nunn GB (1998) Towards a new taxonomy for albatrosses, in G Robertson & R Gales (eds), Albatross biology and conservation. Beatty & Sons, Chipping Norton, Surrey. pp. 13-19.
Robertson CJR & Bell BD (1984) Seabird status and conservation in the New Zealand region, in JP Croxall, PGH Evans & RW Schreiber (eds), Status and conservation of the world's seabirds, ICBP Technical Publication 2, Cambridge. pp. 573-586.
Robertson CJR & van Tets GF (1982) The status of birds at the Bounty Islands. Notornis 29, 311-336.
Robertson CJR & Warham J (1992) Nomenclature of the New Zealand Wandering Albatrosses (Diomedea exulans). Bulletin of the British Ornithologists' Club 112, 74-81.
Robertson CJR, Bell D & Nicholls DG (2000) The Chatham Albatross (Thalassarche eremita): at home and abroad. Notornis 47, 174.
Robertson G, Ashworth P, Ashworth P, Carlyle I & Candy SG (2014) The development and operational testing of an underwater bait setting system to prevent the mortality of albatrosses and petrels in pelagic longline fisheries. Open Journal of Marine Science 5, 1-12.
Robertson G, McNeill M, Smith N, Wienecke B, Candy S & Olivier F (2006) Fast sinking (integrated weight) longlines reduce mortality of White-chinned petrels (Procellaria aequinoctialis) and Sooty shearwaters (Puffinus griseus) in demersal longline fisheries. Biological Conservation 132, 458-471.
Robinson RA, Learmonth JA, Hutson AM, Macleod CD, Sparks TH, Leech DI, Pierce GJ, Rehfisch MM & Crick HQP (2005) Climate change and migratory species. BTO Research Report 414 to the Department of Environment, Department of Environment, Food and Rural Affairs. Research Contract CR0302. British Trust for Ornithology, The Nunnery, UK.
Robinson SA & Copson GR (2014) Eradication of cats (Felis catus) from sub-Antarctic Macquarie Island. Ecological Management and Restoration 15, 34-40.
Rodríguez A, Arcos JM, Bretagnolle V, Dias MP, Holmes ND, Louzao M, Provencher J, Raine AF, Ramírez F, Rodríguez B, Ronconi RA, Taylor RS, Bonnaud E, Borrelle S, Cortés V, Descamps S, Friese VL, Genovart M, Hedd A, Hodum P, Humphries G, Le Corre M, Lebarbenchon C, Martin R, Melvin EF, Montevecchi WA, Pinet P, Polle IL, Ramos R, Russell JC, Ryan PG, Sanz-Aguilar A, Spatz D, Travers M, Votier SC, Wanless R, Woehler E & Chiaradia C (2019) Future directions in conservation research on petrels and shearwaters. Frontiers in Marine Science, 6: 94.
Rodríguez A, Burgan G, Dann P, Jessop R, Negro JJ & Chiaradia A (2014) Fatal attraction of Short-tailed shearwaters to artificial lights. PLoS One 9: e110114.
Rodríguez A, Moffet J, Revoltos A, Wasiak P, McIntosh RR, Sutherland DR, Renwick L, Dann P & Chiaradia A (2017) Light pollution and seabird fledglings: Targeting efforts in rescue programs. Journal of Wildlife Management 81, 734-741.
Roman L, Butcher RG, Stewart D, Hunter S, Jolly M, Kowalski P, Hardesty BD & Lenting B (2020) Plastic ingestion is an underestimated cause of death for southern hemisphere albatrosses. Conservation Letters e:12785.
Ronconi RA, Allard KA & Taylor PD (2015) Bird interactions with offshore oil and gas platforms: review of impacts and monitoring techniques. Journal of Environmental Management 147, 34-45.
Rothschild W (1893) Diomedea bulleri and Thalassogeron salvini. Ibis 5, 572-573.
Rothschild W (1903) Thalassogeron carteri. Bulletin of the British Ornithologists' Club 14, 6.
Rounsevell DE & Brothers NP (1984) The status and conservation of seabirds at Macquarie Island, in JP Croxall, PGH Evans & RW Schreiber (eds), Status and conservation of the world's seabirds. ICBP Technical Publication 2, Cambridge, UK. pp. 587-592.
Roux JP, Jouventin P, Mougin JL, Stahl JC & Weimerskirch H (1983) Un nouvel albatros Diomedea amsterdamensis n. sp. découvert sur I’lle Amsterdam (37°50’S, 77°35’E). L'Oiseau Revue Française d'Ornithologie 53, 1-11.
Rowan MK (1951) The Yellow-nosed Albatross Diomedea chlororhynchos Gmelin, at its breeding grounds in the Tristan da Cunha group. Ostrich 22, 139-155.
Ryan PG (1998) The taxonomic and conservation status of the Spectacled Petrel Procellaria conspicillata. Biological Conservation 131, 575-583.
Ryan PG (2002) Chatham Albatross, Thalassarche eremita: new to Africa. Bulletin of the African Bird Club 9, 43-44.
Ryan P (2007) Field guide to the animals and plants of Tristan da Cunha and Gough Island. Pisces Publications, Newbury, UK.
Ryan PG & Moloney CL (1988) Effect of trawling on bird and seal distributions in the southern Benguela current. Marine Ecology Progress Series 45, 1-11.
Ryan PG, Connell AD & Gardner BD (1988) Plastic ingestion and PCBs in seabirds: is there a relationship? Marine Pollution Bulletin 19, 174-176.
Ryan PG, Phillips RA, Nel DC & Wood AG (2007) Breeding frequency in Grey-headed albatrosses Thalassarche chrysostoma. Ibis 149, 45-52.
Sagar PM (2017) White-capped mollymawk, in CM Miskelly (ed). New Zealand Birds Online. Available on the Internet at: www.nzbirdsonline.org.nz.
Sagar PM & Warham J (1998) Breeding biology of the Southern Buller’s Albatross Diomedea bulleri bulleri at the Snares Islands, New Zealand, in G Robertson & R Gales (eds). Albatross biology and conservation. Beatty & Sons, Chipping Norton, Surrey. pp. 92-98.
Sagar P, Charteris M & Scofield P (2004) Salvin's albatross population size and survival at the Snares Western chain. National Institute of Water & Atmospheric Research, Client Report No. CHC2014-119, Christchurch, New Zealand.
Sagar PM, Molloy J, Weimerskirch H & Warham J (2000) Temporal and age-related changes in survival rates of Southern Buller’s albatrosses (Thalassarche bulleri bulleri) at the Snares, New Zealand, 1948 to 1997. Auk 117, 699-708.
Sagar PM, Stahl JC & Molloy J (2002) The influence of experience, pair bond duration, and partner change on breeding frequency and success in Southern Buller’s mollymawk (Thalassarche bulleri bulleri). Notornis 49, 145-152.
Sagar PM, Stahl JC, Molloy J, Taylor GA & Tennyson AJD (1999) Population size and trends within the two populations of Southern Buller’s albatross Diomedea bulleri bulleri. Biological Conservation 89, 11-19.
Salafsky N, Salzer D, Stattersfield AJ, Hilton-Taylor C, Neugarten R, Butchart SHM, Collen B, Cox N, Master LL, O’Connor S & Wilkie D (2008) A standard lexicon for biodiversity conservation: unified classifications of threats and actions. Conservation Biology 22, 897-911.
Savoca MS, Wohlfeil ME, Ebeler SE & Nevitt GA (2016) Marine plastic debris emits a keystone infochemical for olfactory foraging seabirds. Science Advances 2: e1600395.
Schaffer SA, Costa DP & Weimerskirch H (2001) Behavioural factors affecting foraging effort of breeding Wandering albatrosses. Journal of Animal Ecology 70, 864-874.
Schulz M, Robinson S & Gales R (2005) Breeding of the Grey Petrel (Procellaria cinerea) on Macquarie Island: population size and nesting habitat. Emu 105, 323-329.
Selkirk PM, Seppelt RD & Selkirk DR (1990) Sub-Antarctic Macquarie Island: Environment and Biology. Cambridge University Press, New York.
Sileo L, Sievert PR & Samuel MD (1990) Causes of mortality of albatross chicks at Midway Atoll. Journal of Wildlife Diseases 26, 329-338.
Small C (2005) Regional Fisheries Management Organisations: Their Duties and Performance in Reducing By-catch of Albatrosses and Other Species. BirdLife Global Seabird Programme, BirdLife International, Cambridge, UK.
Senko JF, Nelms SE, Reavis JL, Witherington B, Godley BJ & Wallace BP (2020) Understanding individual and population-level effects of plastic pollution on marine megafauna. Endangered Species Research 43, 234-252.
Sindel BM, Kristiansen PE, Wilson SC, Shaw JD & Williams LK (2017) Managing invasive plants on sub-Antarctic Macquarie Island. The Rangeland Journal 39, 537-549.
Soper T (2004) Antarctica: a guide to the wildlife 4th edition. Globe Pequot Press, Guildford, Connecticut.
Spear LB, Ainley DG & Webb SW (2005) Distribution, abundance, habitat use and behaviour of three Procellaria petrels off South America. Notornis 52, 88-105.
Stagi A, vaz-Ferreira R, Marin Y & Joseph L (1998) The conservation of albatrosses in Uruguayan waters, in G Robertson & R Gales (eds). Albatross biology and conservation. Beatty & Sons, Chipping Norton Surrey. pp. 220-224,
Stahl JC, Bartle JA, Cheshire NG, Petyt C & Sagar PM (1998) Distribution and movements of Buller’s Albatross (Diomedea bulleri) in Australasian seas. New Zealand Journal of Zoology 25, 109-137.
Stephenson J, Budd GM, Manning J & Hansbro P (2005) Major eruption-induced changes to the McDonald Islands, southern Indian Ocean. Antarctic Science 17,259-266.
Stewart FM, Phillips RA, Bartle JA, Craig J & Shooter D (1999) Influence of phylogeny, diet, moult schedule and sex on heavy metal concentrations in New Zealand Procellariiformes. Marine Ecology Progress Series 178, 295-305.
Suazo CG, Oliveira N, Debski I, Mangel JC, Alfaro-Shigueto J, Azócar J, García -Alberto G & Velarde E (2017) Seabird bycatch in purse seine fisheries: status of knowledge and mitigation measures. Agreement for the Conservation of Albatrosses and Petrels, Eighth Meeting of the Seabird Bycatch Working Group, 4-6 September 2017, Wellington, New Zealand, SBWG8 Inf 26.
Sullivan B & Reid T (2002) Seabird interactions/mortality with longliners and trawlers in the Falkland/Malvinas Island waters 2001/02. Commission for the Conservation of Antarctic Marine Living Resources, Second Meeting of the Working Group on Fish Stock Assessment, 7-17 October 2002, Hobart Tasmania, WG-FSA-02/36.
Sullivan BJ & Reid TA (2003) Seabird mortality and trawlers in Falkland Island waters 2002/03. Falklands Conservation, Falklands Islands, UK.
Sullivan BJ, Brickle P, Reid TA, Bone DG & Middleton DAJ (2006a) Mitigation of seabird mortality on factory trawlers: trials of three devices to reduce warp cable strikes. Polar Biology 29, 745-753.
Sullivan BJ, Reid TA & Bugoni L (2006b) Seabird mortality on factory trawlers in the Falkland Islands and beyond. Biological Conservation 131, 495-504.
Tartu S, Angelier F, Wingfield JC, Bustamante P, Labadie P, Budzinski H, Weimerskirch H, Bustnes JO & Chastel O (2015) Corticosterone, prolactin and egg neglect behaviour in relation to mercury and legacy POPs in a long-lived Antarctic bird. Science of the Total Environment 505, 180-188.
Taylor GA (2000) Action plan for seabird conservation in New Zealand. A. Threatened seabirds. Threatened Species Occasional Publication No. 16. Department of Conservation, Wellington, New Zealand.
Techow NMSM, Ryan PG & O’Ryan C (2009) Phylogeography and taxonomy of White-chinned and Spectacled petrels. Molecular Phylogenetics and Evolution 52, 25-33.
Temminck CJ (1828) Albatros sourcils-noirs Diomedea melanophris. Livraison 77, in CJ Temminck & M Laugier de Chartrouse, Nouveau recueil de planches coloriées d'oiseaux pour servir de suite et de complément aux planches enluminées de Buffon 1820-1839. Levrault, Paris. text to pl. 456.
Terauds A, Gales R & Alderman R (2005) Trends in numbers and survival of Black-browed (Thalassarche melanophyrs) and Grey-headed (T. chrysostoma) albatrosses breeding on Macquarie Island. Emu 105, 159-167.
Terauds A, Gales R, Baker GB & Alderman R (2006a) Foraging areas of black-browed and grey-headed albatrosses breeding on Macquarie Island in relation to marine protected areas. Aquatic Conservation: Marine and Freshwater Ecosystems 16, 133-146.
Terauds A, Gales R, Baker GB & Alderman R (2006b) Population and survival trends of Wandering albatrosses (Diomedea exulans) breeding on Macquarie Island. Emu 106, 211-218.
Thiebot JB, Barbraud C, Delord K, Marteau C & Weimerskrich H (2014) Do introduced mammals chronically impact the breeding success of the world’s rarest albatross? Ornithological Science 13, 41-46.
Thiebot JB, Delord K, Barbraud C, Marteau C & Weimerskirch H (2015) 167 individuals versus millions of hooks: bycatch mitigation in longline fisheries underlies conservation of Amsterdam albatrosses. Aquatic Conservation: Marine and Freshwater Ecosystems 26, 674-688.
Thiers L, Delord K, Barbraud C, Phillips RA, Pinaud D & Weimerskirch H (2014) Foraging zones of the two sibling species of giant petrels in the Indian Ocean throughout the annual cycle: implication for their conservation. Marine Ecology Progress Series 499, 233-248.
Thompson D, Sagar P & Torres L (2009) Draft Final Report. A population and distributional study of white-capped albatross (Auckland Islands). Contract Number: POP 2005/02. Conservation Services Programme: Department of Conservation, Wellington, New Zealand.
Thompson DR, Furness RW & Lewis SA (1993) Temporal and spatial variation in the mercury concentrations on some albatrosses and petrels from the sub-Antarctic. Polar Biology 13, 239-244.
Thomson RB, Alderman RL, Tuck GN & Hobday AJ (2015) Effects of climate change and fisheries bycatch on shy albatross (Thalassarche cauta) in southern Australia. PLoS One 10: e0127006.
Thorne LH, Conners MG, Hazen EL, Bograd SJ, Antolos M, Costa DP & Shaffer SA (2016) Effects of El Niño -driven changes in wind patterns on North Pacific albatrosses. Journal of the Royal Society Interface 13: 20160196.
Thost D & Allison I (2005) The climate of Heard Island, in K Green & EJ Woehler (eds), Heard Island: Southern Ocean sentinel. Beatty & Sons, Chipping Norton Surrey.
Thost D & Truffer M (2008) Glacier recession on Heard Island, Southern Indian Ocean. Antarctic and Alpine Research News 40, 199-214.
Tickell WLN (1995) Atlas of southern hemisphere albatrosses. Australasian Seabird Group Newsletter 29, 2-24.
Tickell WLN (2000) Albatrosses. Pica Press, Sussex, England.
Tickell WLN & Pinder R (1975) Breeding biology of the Black-browed Albatross Diomedea melanophris and Grey-headed Albatross D. chrysostoma at Bird Island, South Georgia. Ibis 117, 433-451.
Tomkins RJ (1985) Reproduction and mortality of Wandering albatrosses on Macquarie Island. Emu 85, 40-42.
Townrow K (1988) Sealing sites on Macquarie Island: an archaeological survey, in MR Banks & SJ Smith (eds), Proceedings of the Symposium on Macquarie Island. The Royal Society of Tasmania, Hobart, Tas. pp. 15-26.
Trebilco R, Gales R, Baker G, Terauds A & Sumner D (2008) At sea movement of Macquarie Island giant petrels: relationships with marine protected areas and regional fisheries management organisations. Biological Conservation 141, 2942-2958.
Tremblay-Boyer L & Abraham ER (2020) Increased fisher-reporting of seabird captures during an electronic monitoring trial. New Zealand Aquatic Environment and Biodiversity Report No. 238. Fisheries New Zealand, Wellington. Available on the Internet at: http://www.mpi.govy.nz/news-and-resources/publications.
TSSC (Threatened Species Scientific Committee) (2020) Conservation Advice Thalassarche cauta Shy Albatross. Department of the Agriculture, Water and the Environment, Canberra, ACT. Available on the Internet at: https://www.environment.gov.au/cgi-bin/sprat/public/publicspecies.pl?taxon_id=89224.
Tweedie CE & Bergstrom DM (2000) A climate change scenario for surface air temperature at subantarctic Macquarie Island, in W Davison, C Howard-Williams & P Broady (eds), Antarctic ecosystems: models for wider ecological understanding. New Zealand Natural Sciences, Christchurch, New Zealand. pp. 272-281.
Uhart MM, Gallo L & Quintana F (2018) Review of diseases (pathogen isolation, direct recovery and antibodies) in albatrosses and large petrels worldwide. Bird Conservation International 28, 169-196.
van den Hoff J (2001) Further observations on the cephalopod diet of Wandering albatrosses (Diomedea exulans L.) at Macquarie Island. Emu 101, 169-172.
van den Hoff J (2011) Recoveries of juvenile giant petrels in regions of ocean productivity: potential implications for population change. Ecosphere 2(7), 1-13.
van den Hoff J & Newbery K (2006) Southern Giant Petrels Macronectes giganteus diving on submerged carrion. Marine Ornithology 34, 61-64.
Ventura F, Granadeiro JP, Lukacs PM, Kuepfer A & Catry P (2021) Environmental variability directly affects the prevalence of divorce in monogamous albatrosses. Proceedings of the Royal Society B 208: 20212112.
Voisin JF (1988) Breeding biology of the Northern Giant Petrel Macronectes halli and the Southern Giant Petrel M. giganteus at Ile de la Possession, Iles Crozet, 1966-1980. Cormorant 16, 65-97.
Votier RW, Furness SB, Crane JE, Caldow RWG, Catry P, Ensor K, Hamer KC, Hudson AV, Kalmbach E, Klomp NI, Pfeiffer S, Phillips RA, Prieto I & Thompson DR (2004) Changes in fisheries discard rates and seabird communities. Nature 427, 727-730.
Walker K & Elliott G (1999) Population changes and the biology of the Wandering Albatross (Diomedea antipodensis). Notornis 52, 206-214.
Walker K & Elliott G (2002) Monitoring Gibson's Wandering Albatross, 2001/02. Department of Conservation Science Internal Series 73. Department of Conservation, Wellington, New Zealand.
Walker K & Elliott G (2005) Population changes and the biology of the Wandering Albatross Diomedea exulans gibsoni at the Auckland Islands. Emu 99, 239-247.
Walker JK & Elliott G (2006) At-sea distribution of Gibson's and Antipodean Wandering albatrosses, and relationships with longline fisheries. Nature 53, 265-290.
Walker K & Elliott G (2017) ACAP priority population assessment: Antipodean albatross at Antipodes Island. Agreement on the Conservation of Albatrosses and Petrels. Fourth Meeting of the Population and Conservation Status Working Group, 7-8 September 2017, Wellington, New Zealand, PaCSWG4 Doc 03.
Walker K, Elliott G, Rexer-Huber K, & Parker G (2017) Gibson's Wandering Albatross population study and census 2016/17. Report prepared for the Department of Conservation, Wellington, New Zealand. Available on the Internet at: http://www.doc.govt.nz/Documents/conservation/marine-and-coastal/marine-conservation-services/reports/gibsons-albatross-adams-island2017-finalreport.pdf.
Walsh KJE & Ryan BF (2000) Tropical cyclone intensity increase near Australia as a result of climate change. Journal of Climate 13, 3029-3036.
Wang J, Selleck P, Yu M, Ha W, Rootes C, Gales R, Wise T, Crameri S, Chen H, Broz I, Hyatt A, Woods R, Meeham B, McCullough S & Wang L-A (2014) Novel phlebovirus with zoonotic potential isolated from ticks, Australia. Emerging Infectious Diseases 20, 1040-1043.
Wanless RM (2007) Impacts of the introduced house mouse on the seabirds of Gough Island. PhD Thesis. Percy FitzPatrick Institute. University of Cape Town, Cape Town, South Africa.
Warham J (1990) A checklist of the Procellariiformes. Academic Press, London.
Warham J & Bennington SL (1983) A census of Buller’s Albatross Diomedea bulleri at the Snares Islands, New Zealand. Emu 83, 112-114.
Wastell R, Newton M & Alderman R (2015) On Albatross Island. Focal Printers. Hobart, Tas.
Waugh SM, Doherty PF, Freeman AND, Adams L, Woods GC, Bartle JA & Hedley GK (2006) Demography of Westland petrels (Procellaria westlandica), 1995-2003. Emu 106, 219-226.
Waugh SM, Sagar PM & Paull D (1997) Laying dates, breeding success and annual breeding of Southern Royal albatrosses Diomedea epomophora epomophora at Campbell Island during 1964-69. Emu 97, 194-199.
Waugh SM, Weimerskirch H, Cherel Y & Prince PA (2000) Contrasting strategies of provisioning and chick growth in two sympatrically breeding albatrosses at Campbell Island, New Zealand. Condor 102, 804-813.
Waugh SM, Weimerskirch H, Moore PJ & Sagar PM (1999) Population dynamics of Black-browed and Grey-headed albatrosses Diomedea melanophrys and D. chrysostoma at Campbell Island, New Zealand, 1942-96. Ibis 141, 216-225.
Weimerskirch H (1992) Reproductive effort in long-lived birds: age-specific patterns of condition, reproduction and survival in the Wandering Albatross. Oikos 64, 464-473.
Weimerskirch H (1995) Regulation of foraging trips and incubation routine in male and female Wandering albatrosses. Oecologia 102, 37-43.
Weimerskirch H (1998) Foraging strategies of southern albatrosses and their relationship with fisheries, in G Robertson & R Gales (eds), Albatross biology and conservation. Beatty & Sons, Chipping Norton, Surrey. pp. 168-179
Weimerskirch H (2004) Diseases threaten Southern Ocean albatrosses. Polar Biology 27, 374-379.
Weimerskirch H & Jouventin P (1987) Population-dynamics of the Wandering Albatross, Diomedea-exulans, of the Crozet Islands: causes and consequences of the population decline. Oikos 49, 315-322.
Weimerskirch H & Jouventin J (1998) Changes in population size and demographic parameters of six albatross species breeding in the French sub-Antarctic islands, in G Robertson & R Gales (eds), Albatross biology and conservation. Beatty & Sons, Chipping Norton, Surrey. pp. 84-91.
Weimerskirch H & Robertson G (1994) Satellite tracking of Light-mantled Sooty albatrosses. Polar Biology 14,123-126.
Weimerskirch H & Wilson RP (2000) Oceanic respite for Wandering albatrosses. Nature 406, 955-956.
Weimerskirch H, Akesson S & Pinaud D (2006) Postnatal dispersal of Wandering albatrosses: implications for the conservation of the species. Journal of Avian Biology 37, 23-28.
Weimerskirch H, Bartle JA, Jouventin P & Stahl JC (1988) Foraging ranges and partitioning of feeding zones in three species of southern albatrosses. Condor 90,214-219.
Weimerskirch H, Brothers N & Jouventin P (1997) Population dynamics of Wandering Albatross, Diomedea exulans, and Amsterdam Albatross, D. amsterdamensis in the Indian Ocean and their relationship with long-line fisheries: conservation implications. Biological Conservation 79, 257-270.
Weimerskirch H, Capdeville D & Duhamel G (2000) Factors affecting the number and mortality of seabirds attending trawlers and long-liners in the Kerguelen area. Polar Biology 23, 236-249.
Weimerskirch H, Clobert J & Jouventin P (1987) Survival in five southern albatrosses and its relationship with their life history. Journal of Animal Ecology 56, 1043-1055.
Weimerskirch H, Jouventin P, Mougin JL, Stahl JC & van Beveren M (1985) Banding recoveries and the distribution of seabirds breeding in French Austral and Antarctic Territories. Emu 85, 22-33.
Weimerskirch H, Jouventin P & Stahl JC (1986) Comparative ecology of the six albatross species breeding on the Crozet Islands. Ibis 128, 195-213.
Wienecke B & Robertson G (2002) Seabird and seal: fisheries interactions in the Australian Patagonian toothfish Dissostichus eleginoides trawl fishery. Fisheries Research 54, 252-265.
Wienecke B, Leaper R, Hay I & van den Hoff J (2009) Retrofitting historical data in population studies: Southern Giant petrels in the Australian Antarctic Territory. Endangered Species Research 8, 157-164.
Wiese FK, Montevecchi WA, Davoren GK, Huettmann F, Diamond AW & Linke J (2001) Seabirds at risk around offshore oil platforms in the north-west Atlantic. Marine Pollution Bulletin 42, 1285-1290.
Wilcox C, van Sebille E & Hardesty B (2015) Threat of plastic pollution to seabirds is global, pervasive, and increasing. Proceedings of the National Academy of Sciences 112, 11899-11904.
Woehler EJ (1993) Antarctic seabirds: their status and conservation in the AAT. Royal Australasian Ornithologists Union Conservation Statement No. 9, Supplement to Wingspan 12.
Woehler EJ (2006) Status and conservation of the seabirds of Heard Island and the McDonald Islands, in K Green & EJ Woehler (eds), Heard Island, Southern Ocean Sentinel. Beatty & Sons, Chipping Norton, Surrey. pp. 128-165.
Woehler E & Johnstone G (1988) Banding studies of giant petrels, Macronectes spp. at Macquarie Island. Proceedings of the Royal Society of Tasmania 122, 143-152.
Woehler EJ, Auman HJ & Riddle MJ (2002) Long-term population increase of Black-browed albatrosses Thalassarche melanophrys at Heard Island, 1947/1948 to 2000/2001. Polar Biology 25, 921-927.
Woehler EJ, Martin MR & Johnstone GW (1990) The status of Southern Giant petrels, Macronectes giganteus, at the Frazier Islands, Wilkes Land, East Antarctica. Corella 14, 101-106.
Wood KA (1992) Seasonal abundance and spatial distribution of albatrosses off Central New South Wales. Australian Bird Watcher 14, 207-225.
Woods R (2004) Results of a preliminary disease survey in Shy Albatross (Thalassarche cauta Gould 1841) chicks at Albatross Island, Bass Strait, Tasmania, in Proceedings of the Annual Conference of the Australian Association of Veterinary Conservation Biologists, Canberra, ACT.
Xavier JC, Croxall JP & Reid K (2003) Interannual variation in diets of two albatross species breeding at South Georgia: implications for breeding performance. Ibis 145, 593-610.
Xavier JC, Croxall JP, Trathan PN & Wood AG (2003) Feeding strategies and diets of breeding Grey-headed and Wandering albatrosses at South Georgia. Marine Biology 143, 221-232.
Yeh YM, Huang HW, Dietrich KS & Melvin E (2013) Estimates of seabird incidental catch by pelagic longline fisheries in the South Atlantic Ocean. Animal Conservation 16, 141-152.
Yorio P, Frere E, Gandini P & Schiavini A (2001) Tourism and recreation at seabird breeding sites in Patagonia, Argentina: current concerns and future prospects. Bird Conservation International 11, 231-245.
Young LC & van der Werf EA (2008) Prevalence of avian pox virus and effect on fledging success of Laysan Albatross. Journal of Field Ornithology 79, 93-98.
Zotier R (1990) Breeding ecology of a sub-Antarctic winter breeder: the Grey Petrel Procellaria cinerea on Kerguelen Islands. Emu 90, 180-184.
Žydelis R, Bellebaum J, Österblom H, Vetemaa M, Schirmeister B, Stipniece A, Dagys M, van Eerden M & Garthe S (2009) Bycatch in gillnet fisheries-an overlooked threat to waterbird populations. Biological Conservation 142, 1269-1281.
Žydelis R, Small C & French G (2013) The incidental catch of seabirds in gillnet fisheries: a global review. Biological Conservation 162, 76-88.
Other sources
Agreement for the establishment of the Indian Ocean Tuna Commission, done 25 November 1993, 1927 UNTS 329 (entered into force 27 March 1996). Available on the internet at: https://treaties.un.org/.
Agreement on the Conservation of Albatrosses and Petrels, done 19 June 2001, 2258 UNTS 257 (entered into force 1 February 2004). Available on the internet at: https://treaties.un.org/.
Alderman R (2018) Personal communication by telephone, 5 September 2018, Marine Conservation Program, Department of Primary Industries, Parks, Water and Environment, Tasmania.
Antarctic Marine Living Resources Conservation Act 1981 (Cth). Available on the internet at: http://comlaw.gov.au/.
Antarctic Treaty, done 1 December 1959, 402 UNTS 71 (entered into force 23 June 1961). Available on the internet at: https://treaties.un.org/.
Antarctic Treaty Environment Protection Act 1980 (Cth). Available on the internet at: http://comlaw.gov.au/.
Biodiversity Conservation Act 2016 (NSW). Available on the Internet at: https://www.legislation.nsw.gov.au/.
Biodiversity Conservation Act 2016 (WA). Available on the Internet at: https://www.legislation.wa.gov. au.
Convention concerning the Protection of the World Cultural and Natural Heritage, done 23 November 1972, 1037 UNTS 151 (entered into force 17 December 1975). Available on the internet at: https://treaties.un.org/.
Convention on the Conservation and Management of Highly Migratory Fish Stocks in the Western and Central Pacific Ocean, done 5 September 2000, 2275 UNTS 43 (entered into force 19 June 2004)..Available on the internet at: https://treaties.un.org/.
Convention on the Conservation and Management of High Seas Fishery Resources in the South Pacific Ocean, 14 November 2009, [2012] ATS 28 (entered into force 24 August 2012). Available on the internet at: http://www.austlii.edu.au/au/other/dfat/treaties/.
Convention for the Conservation of Southern Bluefin Tuna, done 10 May 1993, 1819 UNTS 359 (entered into force 20 May 1994). Available on the internet at: https://treaties.un.org/.
Convention on Biodiversity, done 5 June 1992, 1760 UNTS 79 (entered into force 29 December 1993). Available on the internet at: https://treaties.un.org/.
Convention on the Conservation of Antarctic Marine Living Resources, done 20 May 1980, 1329 UNTS 47 (entered into force 7 April 1982). Available on the internet at: https://treaties.un.org/.
Convention on the Conservation of Migratory Species of Wild Animals, done 23 June 1979, 1651 UNTS 333 (entered into force 1 November 1983). Available on the internet at: https://treaties.un.org/.
Environment Protection and Biodiversity Conservation Act 1999 (Cth). Available on the internet at: http://comlaw.gov.au/.
Environment Protection and Biodiversity Conservation Regulations 2000 (Cth). Available on the internet at: http://comlaw.gov.au/.
Environment Protection and Management Ordinance 1987 (Cth). Available on the internet at: http://comlaw.gov.au/.
Fisheries Management Act 1991 (Cth). Available on the internet at: http://comlaw.gov.au/.
Flora and Fauna Guarantee Act 1988 (Vic). Available on the Internet at: http://www.legislation.vic.gov.au/.
Heard Island and McDonald Islands Act 1953 (Cth). Available on the Internet at: http://www.legislation.vic.gov.au/.
Hunt G (Minister for the Environment) (2014) Macquarie Island is declared officially pest free. Media Release, Parliament House, Canberra, 7 April 2014.
McInnes J (2021) Personal communication by email, 15 December 2021, Institute of Marine and Antarctic Studies, University of Tasmania, Hobart, Tasmania.
Nature Conservation Act 1992 (Qld). Available on the Internet at: https://www.legislation.qld.gov.au .
National Parks and Reserves Management Act 2002 (Tas). Available on the internet at: https://www.legislation.tas.gov.au/.
National Parks and Wildlife Act 1972 (SA). Available on the Internet at: https://www.legislation.sa.gov.au/.
Protocol on Environmental Protection to the Antarctic Treaty, done 4 October 1991, 2941 UNTS 9 (entered into force 14 January 1998). Available on the internet at: https://treaties.un.org/.
Southern Indian Ocean Fisheries Agreement, done 7 July 2006, 2835 UNTS 409 (entered into force 21 June 2012). Available on the internet at: https://treaties.un.org/.
Threatened Species Protection Act 1995 (Tas). Available on the internet at: https://www.legislation.tas.gov.au/.
Albatrosses and petrels are tube-nosed seabirds within the Order Procellariiformes (Warham 1990, del Hoyo & Collar 2014). Within the Family Diomedeidae (Table 16) the recovery plan applies to:
- All six great albatross species within the genus Diomedea: Amsterdam Albatross, Antipodean Albatross, Northern Royal Albatross, Southern Royal Albatross, Tristan Albatross and Wandering Albatross. The great albatrosses are large seabirds with long, narrow wings and large pale bills (Onley & Scofield 2007).
- Both sooty albatross species within the genus Phoebetria: Light-mantled Albatross and Sooty Albatross. The sooty albatrosses are medium sized seabirds, with dark bodies, long thin wings and smaller dark bills, when compared to the great albatrosses (Onley & Scofield 2007).
- All 10 mollymawk albatross species within the genus Thalassarche: Atlantic Yellow-nosed Albatross, Black-browed Albatross, Buller’s Albatross, Campbell Albatross, Chatham Albatross, Grey-headed Albatross, Indian Yellow-nosed Albatross, Salvin’s Albatross, Shy Albatross and White-capped Albatross. The mollymawk albatrosses are small to medium sized seabirds, with generally dark backs, shorter and broader wings and smaller bills of varying colour, when compared to the great albatrosses (Onley & Scofield 2007).
Within the Family Procellariidae (Table 16) the plan applies to:
- Both giant-petrel species within the genus Macronectes: Northern Giant Petrel and Southern Giant Petrel. The giant petrels are large seabirds with straight, thin wings and large, bulbous bills (Onley & Scofield 2007).
- Four Procellaria petrel species within the genus Procellaria: Black Petrel, Grey Petrel, Westland Petrel and White-chinned Petrel. These are small seabirds with dark bodies, shorter broader wings and smaller bills, when compared to the giant petrels (Onley & Scofield 2007).
Table 16: Classification of albatross and petrel species to which the recovery plan applies.
ORDER Procellariiformes |
Family Diomedeidae | Genus Diomedea
(great albatrosses) | Amsterdam Albatross Antipodean Albatross Northern Royal Albatross Southern Royal Albatross Tristan Albatross Wandering Albatross |
Genus Phoebetria
(sooty albatrosses) | Light-mantled Albatross Sooty Albatross |
Genus Thalassarche
(small to medium size albatrosses) | Atlantic Yellow-nosed Albatross Black-browed Albatross Buller's Albatross Campbell Albatross Chatham Albatross Grey-headed Albatross Indian Yellow-nosed Albatross Salvin's Albatross Shy Albatross White-capped Albatross |
Family Procellariidae | Genus Macronectes (giant petrels) | Northern Giant Petrel Southern Giant Petrel |
Genus Procellaria (Procellaria petrels) | Black Petrel Grey Petrel Westland Petrel White-chinned Petrel |
For the species considered within the recovery plan, the following species profiles are organised into albatross and petrel species breeding in Australia's jurisdiction, and species that only forage in Australia's jurisdiction. Within each grouping albatross species profiles are provided before those for petrels.
Family: Diomedeidae
Taxonomy
Diomedea exulans Linnaeus 1758 is accepted nomenclature for the Wandering Albatross. Originally Diomedea exulans Linnaeus 1758. There has been considerable debate about the taxonomy for wandering-type albatrosses and whether these should be split into subspecies or recognised at the specific level (ACAP 2012v). Robertson & Nunn (1998) recognised Diomedea exulans at the specific level based on the species’ morphology and genetics, with this nomenclature widely accepted (ACAP 2012v).
Current status of taxon
Environment Protection and Biodiversity Conservation Act 1999 (Commonwealth): Vulnerable
Biodiversity Conservation Act 2016 (Western Australia): Vulnerable
National Parks and Wildlife Act 1972 (South Australia): Vulnerable
Flora and Fauna Guarantee Act 1988 (Victoria): Critically Endangered
Threatened Species Protection Act 1995 (Tasmania): Endangered
Biodiversity Conservation Act 2016 (New South Wales): Endangered
Nature Conservation Act 1992 (Queensland): Vulnerable
IUCN Red list of Threatened Species: Vulnerable
Action Plan for Australian Birds 2020: breeding population Critically Endangered, population visiting Australia Vulnerable
Species description
A large albatross, the Wandering Albatross is approximately 115 cm in length, 6.2-11.2 kg in weight, with a wing length of 61-67 cm, and bill length of 154-172 mm (ACAP 2015, Menkhorst et al. 2017). Tubenosed; separate nostrils on a large, pink plated bill. Combination of white and dark plumage with white head, back and upper wings near body, with black-edged white tail, with white underparts with black trailing edge to underwing and black wingtips (Onley & Scofield 2007, BirdLife International 2018e).
Life history
Breeding locality Jurisdiction |
Macquarie Island | Australia |
Crozet Islands, Kerguelen Islands | France |
Marion Island, Prince Edward Island | South Africa |
South Georgia/Islas Georgia del Sur | Other |
There are 28 breeding sites for the Wandering Albatross that occur on sub-Antarctic island groups of Australia (Macquarie Island), France (Crozet Islands, Kerguelen Islands), South Africa (Marion Island, Prince Edward Island), and other (South Georgia/Islas Georgias del Sur) (ACAP 2012v). The Wandering Albatross is a biennial breeder, when successful, but about 30% of successful and 35% of failed breeders defer breeding beyond the expected year (Croxall et al. 1998). Adults begin arriving at colonies from November. Females lay a single egg in December/January that hatches after a mean incubation period of 11 weeks in February/April (Tickell 2000). The chick remains in the nest for another 9-10 months during which time it may build a new nest for itself, with most chicks fledging in December (Croxall et al. 1990, Tickell 2000). Mean annual breeding success varies between 69-75% annually (Weimerskirch et al. 1997, Croxall et al. 1998, Nel et al.2002). The Wandering Albatross is highly dispersive in all the southern oceans. Juveniles return to breeding colonies between 3-14 years of age, with individuals potentially beginning breeding when 7-10 years of age (Weimerskirch & Jouventin 1987, Pickering 1989, Weimerskirch 1992, Croxall et al. 1998). Generation length is estimated at 22.9 years (Bird et al. 2020).
The Wandering Albatross is present in a very small population of 3-13 breeding pairs on Macquarie Island. Breeding success averages 62.1% ± 3.4% over a 27-year monitoring period. There is a moderate decline in breeding effort -3.17% ± 0.92% over the 27-year period, with an uncertain trend of 0.77 ± 4.44% over the last 10 years (DPIPWE 2021a).
Wandering Albatross mostly feed during daylight (Harper 1987, Phalan et al. 2007). Birds may form large groups (~50 birds) at rich food sources including behind fishing vessels and are voracious scavengers, out-competing all other seabirds for fishing discards and baited hooks (Weimerskirch et al. 1986, Brothers 1991). Birds seize most prey by surface seizing, surface diving to about one metre and rare shallow plunging (Harper 1987, Prince et al. 1994a). Wandering Albatross diet is composed mainly of fish and cephalopod species, with small amounts of other prey (for example, crustaceans and jellyfish) and scavenged species (for example penguins) (Croxall et al. 1988, Cherel & Klages 1998, van den Hoff 2001, Xavier et al. 2003b).
Foraging areas of the Wandering Albatross may vary due to sexual and age-related segregation (BirdLife International 2004). For example, during the breeding season at Crozet Island females forage in subtropical waters to the north of the colony, while males prefer colder, higher latitude waters (Weimerskirch 1995). In non-breeding years, individuals also appear to have a preferred home range, 1500-8500 km from the island, with a similar sexual segregation, females in warmer water than males (Weimerskirch & Wilson 2000).
On dispersal from the colony, juvenile birds frequent subtropical waters of the Indian Ocean and Tasman Sea where wind velocity and productivity are both low; these regions are typically not used by adult birds (Weimerskirch et al. 2006). Breeding adults from Macquarie Island are known to forage in distant oceanic waters over 2000 km away from the island, while non-breeding birds are known to forage in waters north of the island, including New Zealand shelf waters (de la Mare & Kerry 1994, Terauds et al. 2006b). Foraging effort by Wandering Albatross appears to be related to energy acquisition per unit effort, so that food intake levels remain stable, with flight costs the lowest recorded for any seabird (Schaffer et al. 2001).
Species distribution in Australia
The Wandering Albatross nests on Macquarie Island (Figure 1). The species is highly dispersive in all the southern oceans from the edge of the pack ice (68°S), north to at least the Tropic of Capricorn and sometimes beyond. The species’ range approaches 10°S along the western coasts of South America and Africa, and vagrants have been seen off California and in the northern Atlantic. In winter, birds are frequently found north of the Antarctic Convergence (Blakers et al. 1984, Nicholls et al. 1995, 1997, 2000). Comparisons of results of satellite tracking has revealed that distances and patterns of dispersal are variable between breeding stages and populations (BirdLife International 2004, ACAP 2012v). Recent research on the genetic diversity of Wandering Albatross on Macquarie Island has highlighted haplotypes that match Diomedea exulans from a range of breeding sites across their range (McInnes 2021, pers. comm., 15 December 2021). There were also haplotypes detected that closely match those from Diomedea antipodensis (Antipodean Albatross). Further research is needed to determine genetic provenance of these animals.
The Wandering Albatross population on Macquarie Island may have always been small compared to large populations on other similar sized islands (Selkirk et al. 1990). However, circumstantial evidence suggests that Wandering Albatross may have once been numerous on the Island, as seal and penguin oil harvesters that occupied Macquarie Island from 1810 to 1920 used Wandering Albatross as a source of food (Cumpston 1968, Townrow 1988). By the time the Australasian Antarctic Expedition surveyed Macquarie Island in 1913, only one Wandering Albatross pair was left breeding. Once harvesting ceased, the population gradually increased to around 25 breeding pairs by the mid-1960s before declining again (Carrick & Ingham 1970, Terauds et al. 2006b). Macquarie Island may represent marginal habitat for the species because the continental shelf around Macquarie Island is relatively smaller than that adjacent to other sub-Antarctic islands (Selkirk et al. 1990).
A small population of Wandering Albatross may still be present on Heard Island (Kirkwood et al. 1989). A Wandering Albatross pair was brooding a small chick on Heard Island in 1980 (Johnstone 1982). The male had been banded as a non-breeding adult on Macquarie Island in 1967, but the female was not seen. Two old nest mounds found nearby suggests there may have been breeding attempts at the site in previous years.
Population estimates and trends
The breeding population of Wandering Albatross on Macquarie Island is experiencing a moderate decline based on a TRIM analysis (Pannekoek & van Strien 2006) over the last 10 years (DPIPWE 2021a).
Globally, the population trend indicates a population decline for the species exceeding 30% over three generations (BirdLife International 2018e). There were an estimated 9400 breeding pairs in 2021 (ACAP 2022).
Habitat critical to survival of species
Macquarie Island is included on the register of critical habitat for Wandering Albatross under the EPBC Act. The species is limited to 28 breeding sites in Australia (Macquarie Island), France (Crozet Islands, Kerguelen Islands), South Africa (Marion Island, Prince Edward Island), and other (South Georgia/as Georgias del Sur), with the largest population on the Crozet Islands (ACAP 2012v).
Threats
The risk matrix for the Wandering Albatross is provided at Table 17, with the threats occurring in Australia’s jurisdiction highlighted.
Table 17: Wandering Albatross (Diomedea exulans) risk matrix.
Likelihood of occurrence | Consequences |
Not significant | Minor | Moderate | Major | Catastrophic |
Almost certain | Human disturbance: visits to breeding sites | Climate variability and change: variation in Southern Oscillation Index Competition with native species: habitat damage by fur seals Marine pollution: heavy metal contamination Marine pollution: marine plastics ingestion | Fisheries interactions: pelagic longline, trawl, ingestion of discarded hooks | | |
Likely | | | | | |
Possible | | Introduced pest species: predation by cats, house mice, habitat degradation by rabbits. | | | |
Unlikely | Human disturbance: visits to breeding sites | | | | |
Rare or Unknown | | Marine pollution: oil spill contamination | | | |
Note: Threats occurring in Australia's jurisdiction are highlighted in bold.
Figure 1: Modelled Australian distribution of Wandering Albatross (Diomedea exulans).

Family: Diomedeidae
Taxonomy
Phoebetria palbebrata (Forster 1785) is accepted nomenclature for the Light-mantled Albatross. Originally Diomedea palbebrata Forster 1785. The genus Phoebetria was introduced by Reichenbach (1852) and a review by Nichols & Murphy (1914) included the Light-mantled Albatross within that genus as Phoebetria palbebrata. Genetic analyses support this nomenclature (Robertson & Nunn 1998, Nunn et al. 1996) with the nomenclature widely accepted (ACAP 2012m).
Current status of taxon
Environment Protection and Biodiversity Conservation Act 1999 (Commonwealth): not listed
Biodiversity Conservation Act 2016 (Western Australia): not listed
National Parks and Wildlife Act 1972 (South Australia): Vulnerable
Flora and Fauna Guarantee Act 1988 (Victoria): Critically Endangered
Threatened Species Protection Act 1995 (Tasmania): Vulnerable
Biodiversity Conservation Act 2016 (New South Wales): not listed
Nature Conservation Act 1992 (Queensland): Least Concern
IUCN Red list of Threatened Species: Near Threatened
Action Plan for Australian Birds 2020: breeding population Least Concern, population visiting Australia Near Threatened
Species description
A small-medium albatross, the Light-mantled Albatross is approximately 78-90 cm in length, 2.6-3.7 kg in weight, with a wing length of 49-55 cm, and bill length of 98-117 mm (ACAP 2015, Menkhorst et al. 2017). Tubenosed; separate nostrils on a large, black plated bill with bluish bill stripe on lower mandible. Combination of dark and paler grey plumage, except for partial white ring to eye, with head, wings and tail dark, while body paler grey, with slender wings and wedge-pointed tail (Onley & Scofield 2007, BirdLife International 2018i).
Life history
Breeding locality | Jurisdiction |
Heard Island, McDonald Islands, Macquarie Island | Australia |
Crozet Islands, Kerguelen Islands | France |
Antipodes Islands, Auckland Islands, Campbell Islands | New Zealand |
Marion Island, Prince Edward Island | South Africa |
South Georgia/Islas Georgias del Sur | Other |
There are 71 breeding sites for the Light-mantled Albatross that occur on island groups of Australia (Heard Island, McDonald Islands, Macquarie Island), France (Crozet Islands, Kerguelen Islands), New Zealand (Antipodes Islands, Auckland Islands, Campbell Islands), South Africa (Marion Island, Prince Edward Island), and other (South Georgia/Islas Georgias del Sur) (ACAP 2012m). The Light- mantled Albatross is predominantly a biennial breeder, when successful (Jouventin & Weimerskirch 1988, Croxall & Gales 1998). Adults arrive at colonies from early October to mid-September. Females lay a single egg from October/November that hatches after incubation period of 9-10 weeks in December/January (Berruti 1979, DPIPWE 2021a). Fledging of chicks occurs after approximately 4-5 months in May/June (Berruti 1979). Mean annual breeding success varies by location from 15-47% (ACAP 2012m). Light-mantled Albatross fledge one chick, on average, every five years, consequently, this species has one of the lowest reproduction rates for any species of albatross (Weimerskirch et al. 1987). The Light-mantled Albatross has a wide circumpolar range across the southern oceans. Juveniles return to breeding colonies after 7-12 years (Weimerskirch et al. 1987). Generation length is estimated at 21.4 years (Bird et al. 2020).
Light-mantled Albatross feed during the day and at night (Phalan et al. 2007). Birds take prey by surface seizing and plunge diving (Harper 1987). Individual birds may dive to an average of 5 m (with some individuals diving to more than 12 m in pursuit of prey) (Prince et al. 1994a, Harper 1987). Light-mantled Albatrosses do not follow fishing vessels as frequently as other species (Cherel & Klages 1998). Light-mantled Albatross diet is comprised of cephalopods, fish, crustaceans and carrion (including other seabirds) (Cherel & Klages 1998, Green et al. 1998).
Breeding adults forage great distances with Light-mantled Albatrosses breeding at Macquarie Island commonly seen over open waters south and west of Tasmania (Reid et al. 2002). Individuals form Macquarie Island forage up to 2200 km from their nest (Weimerskirch & Robertson 1994). Similarly large foraging ranges have been found at other breeding sites for the species (Akkers 2002, Phillips et al. 2005). Little known about the foraging strategies of Light-mantled Albatross outside the breeding season. The species has a wide, circumpolar range throughout the Southern Ocean and in winter are regular visitors to the pelagic waters of south and south-east Australia (Marchant & Higgins 1990).
Species distribution in Australia
Light-mantled Albatrosses nest on Heard Island, McDonald Islands and Macquarie Island. An indicative distribution map is not available for this non-threatened species. Tracking studies indicate that dispersal is generally between 40-60°S in southern oceans, but may extend to between 20-77°S, and includes southern and sub-Antarctic Australia (Marchant & Higgins 1990, BirdLife International 2004, ACAP 2012m). Birds breeding on Macquarie Island foraged an average 1516 km from the island in sub-Antarctic and Antarctic waters (Weimerskirch & Robertson 1994), however very little is known about the foraging strategies of the Light-mantled Albatross outside the breeding season. The size of the breeding populations of Light-mantled Albatross on Heard Island, McDonald Islands and Macquarie Island before human visitation is unknown. Harvesting of eggs and/or birds may have occurred during the 19th Century while the islands were occupied by sealers (Cumpston 1968, Townrow 1988, Downes 2002).
An island-wide census of the breeding population of Light-mantled Albatross on Macquarie Island was last conducted in 2013 with 2150 ± 300 breeding pairs recorded, with the population currently monitored at three study sites with 79 (67-131) breeding pairs recorded in 2019-20 (DPIPWE 2021a). The population breeding on Heard Island has not been systematically surveyed since 1954 when it was estimated there were 200-500 breeding pairs on the island (Downes et al. 1959), with a later estimate of 500 breeding pairs (minimum) (Woehler 2006). A small population was recorded on McDonald Islands (Johnstone 1980), but its current size is unknown. Visits to McDonald Islands are not permitted, as the location is subject to intermittent volcanic activity (Stephenson et al. 2005, Commonwealth of Australia 2014).
Population estimates and trends
The breeding population of Light-mantled Albatross on Macquarie Island is experiencing a moderate decline based on a TRIM (TRends and Indices for Monitoring data) analysis (Pannekoek & van Strien 2006) over the past 10 years (DPIPWE 2021a), with no estimates available for Heard Island and McDonald Islands.
Global estimates of population change over three generations for the species are subject to considerable uncertainty, due to an absence of comprehensive studies of all breeding populations, with a moderately rapid population decline suspected to be taking place over 100 years (BirdLife International 2018i). There were an estimated 15,900 breeding pairs in 2021, but this figure excluded uncertain estimates of 5000 breeding pairs from the Auckland Islands (ACAP 2022).
Habitat critical to survival of species
Heard Island and McDonald Islands, and Macquarie Island, are subject to management plans that protect these listed world heritage sites and adjacent marine reserves (Parks and Wildlife Service 2006, Commonwealth of Australia 2014). All three breeding populations of Light-mantled Albatross in Australia’s jurisdiction are likely to be important for the long-term persistence of the species within Australia.
The species is limited to 71 breeding sites in Australia (Heard Island, McDonald Islands, Macquarie Island), France (Crozet Islands, Kerguelen Islands), New Zealand (Antipodes Islands, Auckland Islands, Campbell Islands), South Africa (Marion Island, Prince Edward Island), and other (South Georgia/Islas Georgias del Sur), with the largest population at South Georgia/Islas Georgias del Sur (ACAP 2012m).
Threats
The risk matrix for the Light-mantled Albatross is provided at Table 18, with the threats occurring in Australia’s jurisdiction highlighted.
Table 18: Light-mantled Albatross (Phoebetria palbebrata) risk matrix.
Likelihood of occurrence | Consequence |
Not significant | Minor | Moderate | Major | Catastrophic |
Almost certain | | Marine pollution: heavy metal contamination Marine pollution: marine plastics ingestion | Fisheries interactions: pelagic longline | | |
Likely | | | | | |
Possible | | Human disturbance: habitat loss or degradation at breeding sites due to fire Introduced pest species: predation by cats, brown rats, ship rats, house mice, pigs | | | |
Unlikely | | Geological processes: volcanic activity leading to nest abandonment | | | |
Rare or Unknown | | Climate variability and change: sea temperature rise, habitat damage from severe storms, heat stress and degradation of nesting habitat Human disturbance: at breeding sites leading to nest abandonment | | | |
Note: Threats occurring in Australia’s jurisdiction are highlighted in bold.
Family: Diomedeidae
Taxonomy
Thalassarche cauta (Gould 1841) is accepted nomenclature for the Shy Albatross (ACAP 2012q). There has been significant taxonomic debate about the classification of Diomedeidae including, but not limited to, the introduction of the genus Thalassarche by Reichenbach (1852). Originally Diomedea cauta Gould 1841. The Shy Albatross was considered polytypic until it was included in the resurrected genus Thalassarche (Reichenbach 1852) and elevated to the specific level as Thalassarche cauta based on morphological and demographic differences, and genetic analyses (Nunn et al. 1996, Robertson & Nunn 1998). ACAP has concluded on advice from its Taxonomy Working Group that available data warrant recognition of Shy Albatross at the specific level with the nomenclature generally accepted (Double 2006, ACAP 2012q, TSSC 2020).
Current status of taxon
Environment Protection and Biodiversity Conservation Act 1999 (Commonwealth): Endangered
Biodiversity Conservation Act 2016 (Western Australia): Vulnerable
National Parks and Wildlife Act 1972 (South Australia): Vulnerable
Flora and Fauna Guarantee Act 1988 (Victoria): Endangered
Threatened Species Protection Act 1995 (Tasmania): Vulnerable
Biodiversity Conservation Act 2016 (New South Wales): Vulnerable
Nature Conservation Act 1992 (Queensland): Vulnerable
IUCN Red list of Threatened Species: Near Threatened
Action Plan for Australian Birds 2020: Near Threatened
Species description
A medium albatross, the Shy Albatross is approximately 90-110 cm in length, 3.2-5.1 kg in weight, with a wing length of 53-59 cm, and bill length of 122-138 mm (ACAP 2015, Menkhorst et al. 2017). Tubenosed; separate nostrils on a large, pale grey-yellow plated bill with yellowish upper ridge, with yellow tip and yellow at base of bill. Combination of black, grey and white plumage, white head with grey sides and dark eyebrow, white body with black upper wings, black margins and a dark tab intruding into the base of the underwings, and grey-black tail, (Onley & Scofield 2007, BirdLife International 2018p).
Life history
Breeding locality | Jurisdiction |
Albatross Island, Mewstone, Pedra Branca | Australia |
Endemic to Australia with three breeding sites on three Tasmanian islands (Albatross Island, Mewstone, Pedra Branca) (ACAP 2012q). The Shy Albatross is an annual breeder, when successful. Birds are present at the colonies year-round with females laying a single egg predominantly in September, with chicks hatching after 10 weeks in December. Fledging commences when the birds are about four and a half months old in April, although there are minor variations between colonies (Abbott et al. 2006b). Mean annual breeding success averages 43% on Albatross Island with data not available for the other breeding sites (DPIPWE 2021b). The Shy Albatross disperses in the higher latitudes of the Indian Ocean and south-west Atlantic Ocean. Juveniles begin returning to colonies when three years of age, and commence breeding when at least 5-6 years of age, with an average breeding age of nine years (Brothers et al. 1998, Hedd & Gales 2005, Alderman 2012). Generation length is estimated at 20.6 years (Bird et al. 2020).
Shy Albatross usually forage singly or in flocks (~20 birds) (Barton 1979). Birds will also aggregate behind fishing vessels where they are usually able to out-compete all smaller Procellariiformes, all but the great albatrosses (Brothers 1991). Shy albatrosses take fish from surface schools flight feeding, and other prey by surface feeding and surface diving (Barton 1979, Harper et al. 1985, Croxall & Prince 1994). Diving activity occurs mostly during daylight (from 7 am to 10 pm), with the deepest dives (over 7 m) occurring from 10 am to noon (Hedd et al. 1997). Shy Albatross diet is composed mostly of fish and cephalopods, with small amounts of tunicates and crustaceans (Hedd & Gales 2001, McInnes et al. 2020). Prey selection appears to be relatively constant across seasons and years (Hedd & Gales 2001, McInnes et al. 2020). Although natural prey comprises the main diet, scavenging behind fishing vessels is significant food source for a significant proportion of birds (13% generally and up to 29% during some breeding phases) (McInnes et al. 2020).
Shy albatrosses are less oceanic than many other albatross species, are usually found over the continental shelf, and regularly venture close to shore along the coasts of Tasmania and southern Australia (Brothers et al. 1998, Hedd et al. 2001, Reid et al. 2002). The range also extends to southern Africa and into the southern Atlantic Ocean (Barton 1979, Blakers et al. 1984, Tickell 1995, Reid et al. 2002, BirdLife International 2004, Abbott et al. 2006a, Jiménez et al. 2015). During the breeding season, adults forage close to their colonies, usually within 300 km in continental shelf waters (Hedd et al. 2001). Sub-adult dispersal appears to be colony specific with birds from Albatross Island remaining in southern Australian waters, and some birds from the Mewstone dispersing between South Africa and New Zealand, noting that there are no records of immature birds banded at Pedra Branca being recovered away from that colony (Brothers et al. 1997).
Species distribution in Australia
The Shy Albatross only nests on Albatross Island, the Mewstone and Pedra Branca off Tasmania (Figure 2). Tracking studies, band recoveries and genetic identification of Shy Albatross bycaught during fishing operations indicate that adult birds predominantly occur in waters adjacent to Tasmania and southern Australia (Brothers et al. 1997, Abbott et al. 2006a). The range of juvenile birds extends however across the Indian Ocean to southern Africa and potentially the south-western Atlantic Ocean (Barton 1979, Alderman 2012, Jiménez et al. 2015).
Significant harvesting of adult Shy Albatross for their feathers and eggs occurred at Albatross Island during the 1800s with the subpopulation declining from about 20,000 birds to about 300 birds by the end of that century, with the population slowly recovering since then (Green 1974, Johnstone et al. 1975, Brooke 2004).
Shy Albatross is subject to ongoing long-term monitoring on Albatross Island, with aerial surveys used for the Mewstone and Pedra Branca, as access to these locations is restricted for logistical and safety reasons. The number of breeding pairs in 2019/20 was estimated at 5385 (4910-5947) on Albatross Island and 81 (± 1) on Pedra Branca, with around 10,000 (± 200) pairs estimated on the Mewstone in 2014/15 (DPIPWE 2021b).
Population estimates and trends
The breeding population of Shy Albatross is considered stable on Albatross Island based on a TRIM analysis (Pannekoek & van Strien 2006) over the past 10 years, at the Mewstone the trend is uncertain, with Pedra Branca experiencing a steep decline over the same period (DPIPWE 2021b). Population models predict a decline in the number of breeding females in the Albatross Island subpopulation over three generations, but discrepancies exist between the predictions and the most recent empirical data, meaning there is uncertainty around the extent of the decline (TSSC 2020). There is a predicted decline in the Pedra Branca subpopulation linked to interspecies competition with the Australasian Gannet and storm surges (Alderman et al. 2011, Alderman 2018, ACAP 2012q, TSSC 2020). There were an estimated 15,000 breeding pairs in 2021 (ACAP 2022).
Eligibility for listing in the Endangered category under the EPBC Act reflects the restricted area of occupancy of the species; the precarious geographic distribution for the survival of the species, because its number of locations is restricted; a projected decline in the number of mature individuals for the Albatross Island subpopulation, and inferred declines for the Pedra Banca and Mewstone subpopulations. Inclusion of the Shy Albatross in the IUCN Red List Near Threatened category reflects the lack of negative trend data for the larger Mewstone population, which would enable the species to achieve qualification to a higher conservation status. The Action Plan for Australian Birds 2020 (Garnett & Baker 2021) highlights that there is need for further empirical data to confirm whether and to what extent the Shy Albatross population is declining.
Habitat critical to survival of species
The Tasmanian islands of Albatross Island, the Mewstone and Pedra Branca are included on the register of critical habitat for Shy Albatross under the EPBC Act. The species is limited to these three breeding sites in Australia, with the largest population on the Mewstone (ACAP 2012q).
Threats
The risk matrix for the Shy Albatross is provided at Table 19, with the threats occurring in Australia’s jurisdiction highlighted.

Table 19: Shy Albatross (Thalassarche cauta) risk matrix.
Likelihood of occurrence | Consequences |
Not significant | Minor | Moderate | Major | Catastrophic |
Almost certain | Dependence on fisheries discards | Marine pollution: marine plastics ingestion | Climate variability and change: sea temperature rise, habitat damage from severe storms, heat stress and degradation of nesting habitat from higher temperatures Competition with native species: for nesting material by Australasian Gannets affecting Pedra Branca Fisheries interactions: pelagic longline, demersal longline, trawl | Climate variability and change: habitat damage from severe storms affecting Pedra Branca | |
Likely | | Human disturbance: visits to breeding sites, boat traffic and overflights | Disease: tick-borne Phlebovirus outbreaks | | |
Possible | | | | | |
Unlikely | | | | | |
Rare or Unknown | | Human disturbance: wind farm infrastructure | | | |
Note: Threats occurring in Australia’s jurisdiction are highlighted in bold.
Figure 2: Modelled Australian distribution of Shy Albatross (Thalassarche cauta).

Family: Diomedeidae
Taxonomy
Thalassarche chrysostoma (Forster 1785) is accepted nomenclature for the Grey-headed Albatross. There has been significant taxonomic debate about the classification of Diomedeidae including, but not limited to, the introduction of the genus Thalassarche by Reichenbach (1852). Originally Diomedea chrysostoma Forster 1785. The Grey-headed Albatross was included in the resurrected genus Thalassarche (Reichenbach 1852) as Thalassarche chrysostoma based on demographic differences and genetic analyses (Nunn et al. 1996, Robertson & Nunn 1998) with the nomenclature widely accepted (ACAP 2012j).
Current status of taxon
Environment Protection and Biodiversity Conservation Act 1999 (Commonwealth): Endangered
Biodiversity Conservation Act 2016 (Western Australia): Vulnerable
National Parks and Wildlife Act 1972 (South Australia): Vulnerable
Flora and Fauna Guarantee Act 1988 (Victoria): Endangered
Threatened Species Protection Act 1995 (Tasmania): Endangered
Biodiversity Conservation Act 2016 (New South Wales): not listed
Nature Conservation Act 1992 (Queensland): Endangered
IUCN Red list of Threatened Species: Endangered
Action Plan for Australian Birds 2020: breeding population Endangered, population visiting Australia Endangered
Species description
A small-medium albatross, the Grey-headed Albatross is approximately 70-85 cm in length, 2.6-3.8 kg in weight, with a wing length of 50-55 cm, and bill length of 109-121 mm (ACAP 2015, Menkhorst et al. 2017). Tubenosed; separate nostrils on a large, mostly black plated bill, with yellow upper and lower ridge that narrows at base of bill. Combination of dark, grey and white plumage, with grey head and white body, with white eye-patch, with dark upper wings and back, and tail, and leading edge of underwings and wing tips (Onley & Scofield 2007, BirdLife International 2018r).
Life history
Breeding locality | Jurisdiction |
Macquarie Island | Australia |
Islas Diego Ramirez, Islas Ildefonso | Chile |
Crozet Islands, Kerguelen Islands | France |
Campbell Island | New Zealand |
Prince Edward Islands | South Africa |
South Georgia/Islas Georgias del Sur | Other |
There are 29 breeding sites for the Grey-headed Albatross that occur on island groups of Australia (Macquarie Island), Chile (Islas Diego Ramirez, Islas Ildefonso), France (Crozet Islands, Kerguelen Islands), New Zealand (Campbell Island), South Africa (Prince Edward Islands), and other (South Georgia/Islas Georgias del Sur) (ACAP 2012j). The Grey-headed Albatross is predominantly a biennial breeder, when successful (Ryan et al. 2007). Adults arrive at colonies from early September to early October (Weimerskirch et al. 1986, Brooke 2004, Terauds et al. 2005). Females lay a single egg in October that hatches after incubation period of 10 weeks in December (Weimerskirch et al. 1986, Terauds et al. 2005, DPIPWE 2021a). Fledging of chicks occurs after approximately 4-5 months in late April/May (Tickell & Pinder 1975, Weimerskirch et al. 1986, Terauds et al. 2005, DPIPWE 2021a). Mean annual breeding success varies by location from 34-72% (ACAP 2012j). The Grey-headed Albatross has a wide circumpolar range across the southern oceans. Juveniles return to breeding colonies after at least three years and commence breeding on average between 7-13 years depending on location (ACAP 2012j). Generation length is estimated at 22.7 years (Bird et al. 2020).
Grey-headed Albatross take most prey by surface-seizing and surface-plunging (Wood 1992, Prince et al. 1994a). Birds can dive to at least 6 m and remain swimming below the surface for up to 11 seconds in search of prey (Prince et al. 1994a). Diet is composed mostly of fish and cephalopods, with small amounts of other species (Waugh et al. 2000, Cherel et al. 2002, Xavier et al 2003a, 2003b).
Foraging areas of the Grey-headed Albatross may vary due to sexual segregation (Phillips et al. 2004). During the breeding period adults will travel hundreds or thousands of kilometres from the colony, generally to forage waters within or south of the Antarctic Polar Frontal Zone (Prince et al. 1998, Weimerskirch et al. 1988). Grey-headed albatrosses breeding at Macquarie Island typically forage in waters south of the island, frequently travelling into the Southern Ocean (BirdLife International 2004, Terauds et al. 2006a). Non-breeding adults and immature birds disperse widely over the Southern Ocean and may exhibit a circumpolar winter distribution (Croxall et al. 2005). The Grey-headed Albatross is a regular visitor to Australia and New Zealand, especially in winter, particularly south and west of Tasmania (Blakers et al. 1984, Reid et al. 2002).
Species distribution in Australia
The Grey-headed Albatross nests on Macquarie Island (Figure 3). Tracking studies indicate that dispersal is circumpolar in higher latitudes in southern oceans between 35-65°S including southern and sub-Antarctic Australia, and the AAT (Weimerskirch et al. 1986, Weimerskirch et al. 1988, BirdLife International 2004, ACAP 2012j). A more northerly distribution between 39-51°S is apparent during the austral winter (Marchant & Higgins 1990).
Birds breeding on Macquarie Island predominantly forage in sub-Antarctic and Antarctic waters south of the island (Terauds et al. 2006a), however very little is known about the foraging strategies of Grey Albatross outside the breeding season. The size of the breeding populations of Grey-headed Albatross on Macquarie Island before human visitation is unknown. Harvesting of eggs and/or birds may have occurred during the 19th century while the island was occupied by sealers (Cumpston 1968, Townrow 1988). An island-wide census of the breeding population of Grey-headed Albatross on Macquarie Island was conducted in 2019/20 with 113 (58-114) breeding pairs recorded (DPIPWE 2021a).
Population estimates and trends
The breeding population of Grey-headed Albatross on Macquarie Island is experiencing a moderate increase based on a TRIM analysis (Pannekoek & van Strien 2006) over the past 10 years (DPIPWE 2021a).
Globally, the population is experiencing a very rapid decline over three generations (BirdLife International 2018r). There were an estimated 80,850 breeding pairs in 2020 (ACAP 2022).
Habitat critical to survival of species
Macquarie Island is included on the register of critical habitat for the Grey-headed Albatross under the EPBC Act. The species is limited to 29 breeding sites that occur on island groups of Australia (Macquarie Island), Chile (Islas Diego Ramirez, Islas Ildefonso), France (Crozet Islands, Kerguelen Islands), New Zealand (Campbell Island), South Africa (Prince Edward Islands), and other (South Georgia/Islas Georgias del Sur), with the largest population on South Georgia/as Georgias del Sur (ACAP 2012j).
Threats
The risk matrix for the Grey-headed Albatross is provided at Table 20, with the threats occurring in Australia’s jurisdiction highlighted.
Table 20: Grey-headed Albatross (Thalassarche chrysostoma) risk matrix.
Likelihood of occurrence | Consequences |
Not significant | Minor | Moderate | Major | Catastrophic |
Almost certain | | Climate variability and change: variation in Southern Oscillation Index, sea temperature rise Marine pollution: marine plastics ingestion | Fisheries interactions: pelagic longline, demersal longline Introduced pest species: predation by cats, black rats, house mice | | |
Likely | | | | | |
Possible | | | | | |
Unlikely | | | | | |
Rare or Unknown | | Climate variability and change: habitat damage from severe storms, heat stress and degradation of nesting habitat Disease: avian cholera | | | |
Note: Threats occurring in Australia’s jurisdiction are highlighted in bold.
Figure 3: Modelled Australian distribution of Grey-headed Albatross (Thalassarche chrysostoma).
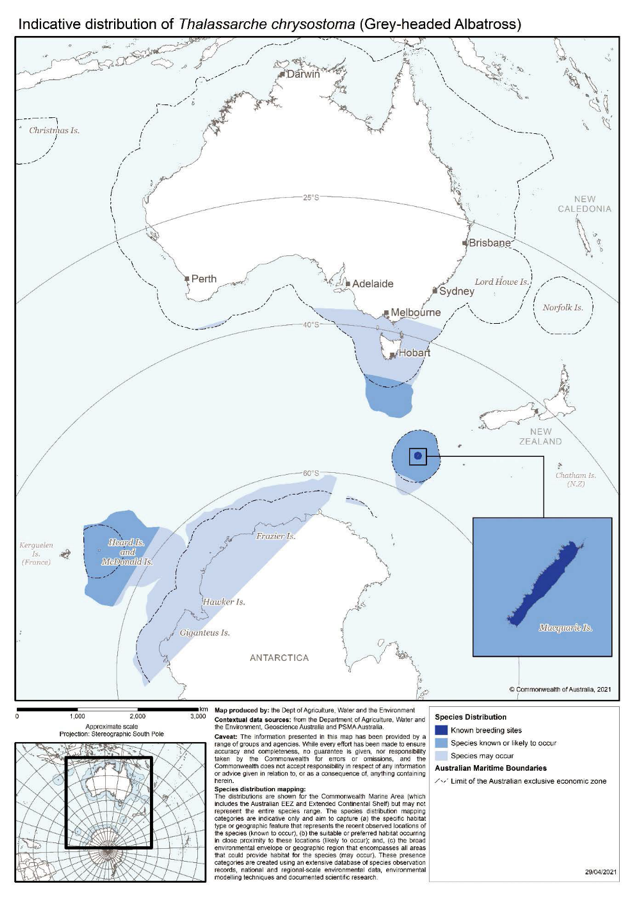
Family: Diomedeidae
Taxonomy
Thalassarche melanophris (Temminck 1828) is accepted nomenclature for the Black-browed Albatross. There has been significant taxonomic debate about the classification of Diomedeidae including, but not limited to, the introduction of the genus Thalassarche by Reichenbach (1852). Originally Diomedea melanophris Temminck 1828. The Black-browed Albatross was considered polytypic until it was included in the resurrected genus Thalassarche (Reichenbach 1852) at the specific level as Thalassarche melanophris based on genetic analyses (Robertson & Nunn 1998) with the nomenclature generally accepted (ACAP 2012d).
Current status of taxon
Environment Protection and Biodiversity Conservation Act 1999 (Commonwealth): Vulnerable
Biodiversity Conservation Act 2016 (Western Australia): Endangered
National Parks and Wildlife Act 1972 (South Australia): not listed
Flora and Fauna Guarantee Act 1988 (Victoria): not listed
Threatened Species Protection Act 1995 (Tasmania): Endangered
Biodiversity Conservation Act 2016 (New South Wales): Vulnerable
Nature Conservation Act 1992 (Queensland): Least Concern
IUCN Red list of Threatened Species: Least Concern
Action Plan for Australian Birds 2020: breeding population Least Concern, population visiting Australia Least Concern
Species description
A small-medium albatross, the Black-browed Albatross is approximately 80-95 cm in length, 2.8-4.3 kg in weight, with a wing length of 51-56 cm, and bill length of 114-122 mm (ACAP 2015, Menkhorst et al. 2017). Tubenosed; separate nostrils on a large, mostly orange plated bill, with reddish tip. Combination of black and white plumage, with white head and body, with black eyebrow and dark iris, with black upper wings and back, and tail, and extensive leading edge of underwings and wing tips (Onley & Scofield 2007, BirdLife International 2018u).
Life history
Breeding locality | Jurisdiction |
Heard Island, McDonald Islands, Macquarie Island | Australia |
Isla Diego de Almagro, Islas Diego Ramirez, Islas Ildefonso, Isolete Albatross, Isloete Leonard, Islotes Evangelistas | Chile |
Crozet Islands, Kerguelen Islands | France |
Antipodes Islands, Campbell Islands | New Zealand |
Falkland Islands/Islas Malvinas, South Georgia/Islas Georgias del Sur | Other |
There are 69 breeding sites for the Black-browed Albatross that occur on island groups of Australia (Heard Island, McDonald Islands, Macquarie Island), Chile (Isla Diego de Almagro, Islas Diego Ramirez, Islas Ildefonso, Isolete Albatross, Isloete Leonard, Islotes Evangelistas) France (Crozet Islands, Kerguelen Islands), New Zealand (Antipodes Islands, Auckland Islands, Campbell Islands), and other (Falkland Islands/Islas Malvinas, South Georgia/Islas Georgias del Sur) (ACAP 2012d). The Black-browed Albatross is an annual breeder when successful, with a significant level of non-breeding in the subsequent year for successful (25%) and unsuccessful breeders (23%) (Croxall et al. 1998). The breeding cycle varies by location. For example, adults arrive at Macquarie Island from early September, with females laying a single egg from late September/October that hatches after incubation period of 9-10 weeks in December/January, with fledging of chicks occurring after approximately 4 months in April/May (Terauds et al. 2005). Mean annual breeding success varies by location from 27-66%, depending on location (ACAP 2012d). The Black-browed Albatross has a wide circumpolar range across the southern oceans. Juveniles return to breeding colonies after at least two years with breeding commencing when birds are 8-10 years of age (Prince et al. 1994b). Generation length is estimated at 23.6 years (Bird et al. 2020).
Black-browed Albatross take most prey by surface-seizing and surface-plunging and pursuit diving, and are frequent fishing vessel followers. Some individuals are capable of remaining submerged for over 50 seconds in pursuit of prey (Guilford et al. 2022). Individual birds reach a maximum depth of 19 m while foraging (Guilford et al. 2022). All individuals dived to more than one metre, indicating that diving is a common mode of capturing prey (Harper 1987, Guilford et al. 2022). Black-browed Albatross diet is composed mostly of fish, cephalopods, jellyfish and scavenged species, with small amounts of other species (Cherel et al. 2002, Xavier et al. 2003a, 2003b, McInnes et al. 2017). For example, at the Kerguelen Islands Black-browed Albatross forage on cephalopods, fish and penguins in roughly equal proportions (Cherel et al. 2002). The diet of fish species includes species discarded from fishing operations, with the presence of fish hooks and fish bait species indicating a strong association with fisheries in southern Chile, Falkland Islands/Islas Malvinas and Kerguelen Islands (Arata & Xavier 2003, McInnes et al. 2017b).
Foraging areas of Black-browed Albatross are extensive with birds accessing Antarctic and sub-Antarctic shelf waters proximate to their breeding islands during the breeding season, but ranging widely when not breeding and favouring the warmer coastal or shelf waters of Australia, New Zealand, South Africa and South America (Weimerskirch et al. 1985, 1986). Black-browed Albatross in Australia’s jurisdiction forage along the southern coastline from Perth to Brisbane (Blakers et al. 1984, Marchant & Higgins 1990, Reid et al. 2002). Sub-adults remain in Australian waters all year round with immature birds forming the preponderance (99%) of Black-browed Albatross seen in south-eastern Australian waters between October and January (Reid et al. 2002).
Species distribution in Australia
Black-browed Albatross nests on Heard Island, McDonald Islands and Macquarie Island (including Bishop and Clerk Islets) (Figure 4). Tracking studies indicate that dispersal is circumpolar from subtropical waters to the Antarctic ice-edge and includes southern and sub-Antarctic Australia, and the AAT (BirdLife International 2004, Terauds et al. 2005, ACAP 2012d), with birds breeding on Macquarie Island generally foraging in the adjacent exclusive economic zone (Terauds et al. 2006a).
The size of the breeding populations of Black-browed Albatross on Heard Island, McDonald Islands and Macquarie Island before human visitation is unknown. Harvesting of eggs and/or birds may have occurred during the 19th Century while the islands were occupied by sealers (Cumpston 1968, Townrow 1988, Downes 2002). Black-browed Albatross is subject to ongoing long-term monitoring on Macquarie Island with 47 (range 42-49) breeding pairs estimated in 2019/20, with long-term breeding success averaging 45% (DPIPWE 2021a). The population breeding on Heard Island has not been systematically surveyed since 2000 when there were around 600 breeding pairs on the island (Woehler et al. 2002). The population on McDonald Islands was last estimated in 1981 when 82-89 breeding pairs were recorded (Keage & Johnstone 1982). Visits to McDonald Islands are not permitted, as the location is subject to intermittent volcanic activity (Stephenson et al. 2005, Commonwealth of Australia 2014). Bishop and Clerk Islets have only been visited on three occasions with 141 active nests identified on Bishop Islet when the site was last visited in 1993 (Brothers & Ledingham 2008).
Population estimates and trends
The breeding population of Black-browed Albatross on Macquarie Island is considered stable based on a TRIM analysis (Pannekoek & van Strien 2006) over the past 10 years (DPIPWE 2021a), with no estimates available for Bishop and Clerk Islets, Heard Island and McDonald Islands.
Globally, the population appears to be increasing over three generations (BirdLife International 2018u). There were an estimated 689,400 breeding pairs in 2020 (ACAP 2022).
Habitat critical to survival of species
Heard Island and McDonald Islands, and Macquarie Island, are subject to management plans that protect these listed world heritage sites and adjacent marine reserves (Parks and Wildlife Service 2006, Commonwealth of Australia 2014). All breeding populations of Black-browed Albatross in Australia’s jurisdiction are likely to be important for the long-term persistence of the species within Australia. The species is limited to 65 breeding sites in Australia (Heard Island, McDonald Islands, Macquarie Island), Chile (Isla Diego de Almagro, Islas Diego Ramirez, Islas Ildefonso, Islote Albatross, Islote Leonard, Islotes Evangelistas) France (Crozet Islands, Kerguelen Islands), New Zealand (Antipodes Islands, Auckland Islands, Campbell Islands), and other (Falkland Islands/as Malvinas), South Georgia/Islas Georgias del Sur), with the largest population at the Falkland Islands/Islas Malvinas) (ACAP 2012d).
Threats
The risk matrix for the Black-browed Albatross is provided at Table 21, with the threats occurring in Australia’s jurisdiction highlighted.
Table 21: Black-browed Albatross (Thalassarche melanophris) risk matrix.
Likelihood of occurrence | Consequences |
Not significant | Minor | Moderate | Major | Catastrophic |
Almost certain | | Climate variability and change: sea surface temperature rise Introduced pest species: predation by cats | Fisheries interactions: pelagic longline, demersal longline, trawl | | |
Likely | | | | | |
Possible | | Geological processes: volcanic activity leading to nest abandonment | | | |
Unlikely | | | | | |
Rare or Unknown | | Climate variability and change: habitat damage from severe storms, heat stress and degradation of nesting habitat Disease: avian cholera | | Climate variability and change: habitat damage from severe storms affecting Bishop and Clerk Islets | |
Note: Threats occurring in Australia's jurisdiction are highlighted in bold.
Figure 4: Modelled Australian distribution of Black-browed Albatross (Thalassarche melanophris).

Family: Diomedeidae
Taxonomy
Macronectes giganteus (Gmelin 1789) is accepted nomenclature for the Southern Giant Petrel. Originally Procellaria gigantea Gmelin 1789. The Southern Giant Petrel was considered polytypic with Macronectes halli (Northern Giant Petrel) until Bourne & Warham (1966) proposed recognition at the specific level, based on morphology and demographic differences, a view also supported by genetic differences (Nunn & Stanley (1998), despite some hybridisation (Hunter 1983). ACAP has concluded on advice from its Taxonomy Working Group that available data warrant recognition of the Southern Giant Petrel at the specific level with the nomenclature widely accepted (Brooke et al. 2007, ACAP 2012s).
Current status of taxon
Environment Protection and Biodiversity Conservation Act 1999 (Commonwealth): Endangered
Biodiversity Conservation Act 2016 (Western Australia): not listed
National Parks and Wildlife Act 1972 (South Australia): Vulnerable
Flora and Fauna Guarantee Act 1988 (Victoria): Endangered
Threatened Species Protection Act 1995 (Tasmania): Vulnerable
Biodiversity Conservation Act 2016 (New South Wales): Endangered
Nature Conservation Act 1992 (Queensland): Endangered
IUCN Red list of Threatened Species: Least Concern
Action Plan for Australian Birds 2020: breeding population Least Concern, population visiting Australia Least Concern
Species description
A large petrel, the Southern Giant Petrel is approximately 87 cm in length, 3.5-5.5 kg in weight, with a wing length of 46-56 cm, and bill length of 84-111 mm (ACAP 2015, Menkhorst et al. 2017). Tubenosed; separate nostrils on a bulbous, pink plated bill with pale green tip. Combination of grey-brown and white plumage, pale head with dark eye, dark body with grey-brown upper wings, and paler underwings, with occasional all white forms with few grey-brown feathers (Onley & Scofield 2007, BirdLife International 2018g).
Life history
Breeding locality | Jurisdiction |
Adélie Land, Antarctic Peninsula, South Orkney Islands, South Shetland Islands | Antarctica |
Isla Arce, Isla de los Estados, Isla Gran Robredo, Isla Observatorio | Argentina |
Australian Antarctic Territory, Heard Island, McDonald Islands, Macquarie Island | Australia |
Isla Noir, Islas Diego Ramirez | Chile |
Crozet Islands, Kerguelen Islands | France |
Prince Edward Islands | South Africa |
Gough Island | United Kingdom |
Falkland Islands/Islas Malvinas, South Georgia/Islas Georgias del Sur, South Sandwich Islands/Islas Sandwich del Sur | Other |
There are 193 breeding sites for the Southern Giant Petrel that occur in Antarctica (Adélie Land, Antarctic Peninsula, South Orkney Islands, South Shetland Islands), and on island groups of Argentina (Isla Arce, Isla de los Estados, Isla Gran Robredo, Isla Observatorio), Australia (AAT, Heard Island, McDonald Islands, Macquarie Island), Chile (Isla Noir, Islas Diego Ramirez), France (Crozet Islands, Kerguelen Islands), South Africa (Prince Edward Islands), and United Kingdom (Gough Island), and other (Falkland Islands/Islas Malvinas, South Georgia/Islas Georgias del Sur, South Sandwich Islands/Islas Sandwich del Sur) (ACAP 2012s). The majority of the Southern Giant Petrel population breeds annually, however a significant proportion (20-40%) of non-breeding is evident each year during ‘sabbatical’ periods that average 1.4 years (Voisin 1988). The start of the breeding cycle varies by location. For example, adults arrive at Macquarie Island in August/September, with females laying a single egg from late August to early September that hatches after incubation period of about 9 weeks in October to mid-November, with fledging of chicks occurring after approximately 4 months from late January to late March (Patterson et al. 2008, DPIPWE 2021a). Mean annual breeding success varies by location depending on location, with breeding success estimated at 50 ± 10% on Macquarie Island (ACAP 2012s, DPIPWE 2021a). The Southern Giant Petrel has a wide circumpolar range across the southern oceans including Antarctica. Juveniles return to Macquarie Island to commence breeding when birds are 9-11 years of age (Woehler & Johnstone 1988). Generation length is estimated at 20.6 years (Bird et al. 2020).
Southern Giant Petrel forage at sea, and also scavenge on land. Birds take prey at sea by surface seizing, surface filtering, surface diving, and surface plunging (Harper 1987). Individual birds may dive up to 3 metres in pursuit of prey or carrion (Harper 1987, van den Hoff & Newbery 2006). They are aggressive opportunists scavenging prey from other smaller birds (Harper et al. 1985, Harper 1987). During breeding periods Southern Giant Petrel prey mainly on cephalopods and fish at sea, and also prey on penguin chicks on land and scavenge seal or penguin carrion (Harper 1987, Le Bohec et al. 2003). Incidents of depredation of adult Atlantic Yellow-nosed Albatross have been reported from Gough Island (Risi et al. 2021).
Southern Giant Petrel range widely throughout the southern oceans with foraging areas varying due to sexual segregation (Patterson & Hunter 1999, Patterson et al. 2008, Trebilco et al. 2008). Foraging range of breeding adults may vary markedly. At Macquarie Island, during the incubation stage adults undertook long trips of up to 19 days south of Macquarie Island, often covering thousands of kilometres to areas south of the Antarctic Circumpolar Current (Trebilco et al. 2008). As the chicks hatched, the length of the foraging trips decreased and birds spent more time close to Macquarie Island. Throughout the colder months, immature birds and most adults disperse widely. Some adults are mainly sedentary, remaining close to their breeding islands throughout the year. Nonetheless, numbers diminish at all sites over winter, with the Antarctic colonies being almost completely abandoned. The waters off south-east Australia may be particularly important wintering grounds with most Southern Giant Petrel (over 80%) sighted off south-east Australia being immature birds (Marchant & Higgins 1990, Reid et al. 2002).
Species distribution in Australia
Southern Giant Petrel nests on Heard Island, McDonald Islands and Macquarie Island, as well as in the AAT (Figure 5). Tracking and banding studies indicate that dispersal is circumpolar from the Tropic of Capricorn to Antarctica and includes southern and sub-Antarctic Australia, and the AAT (BirdLife International 2004, van den Hoff 2011, ACAP 2012s). During the breeding cycle, birds on Macquarie Island generally forage southwards to the Antarctic ice edge or eastwards to South America (Patterson & Hunter 1999, Trebilco et al. 2008).
The size of the breeding populations of Southern Giant Petrel on Heard Island, McDonald Island and Macquarie Island before human visitation is unknown. Harvesting of eggs and/or birds may have occurred during the 19th Century while the islands were occupied by sealers (Cumpston 1968, Townrow 1988, Downes 2002). Southern Giant Petrel is subject to ongoing long-term monitoring on Macquarie Island with the number of breeding pairs estimated to be between 1450-2180 pairs in 2019/20 based on estimated numbers of pre-fledging chicks, and long-term breeding success estimated at 50 ± 10% (DPIPWE 2021a). The population breeding on Heard Island has not been systematically surveyed since 1988 when there were around 3150 breeding pairs on the island (Kirkwood et al. 1995). The population on McDonald Island was last estimated in 1979 when around 1400 breeding pairs were recorded (DSEWPC 2011b). Visits to McDonald Islands are not permitted, as the location is subject to intermittent volcanic activity (Stephenson et al. 2005, Commonwealth of Australia 2014).
Southern Giant Petrel colonies are found in the AAT: 237 breeding pairs were estimated at the Frazier Islands, Wilkes Land in 2011 (ATCM 2013), four occupied nests occurred at Giganteus Island, Mac.Robertson Land in 2007 (ATCM 2015), and about 23 breeding pairs were estimated at Hawker Island, Princess Elizabeth Land in 2014 (ATCM 2016).
Population estimates and trends
The breeding population of Southern Giant Petrel on Macquarie Island is considered stable based on a TRIM analysis (Pannekoek & van Strien 2006) over the past 10 years (DPIPWE 2021a), with no estimates available for Heard Island and McDonald Islands or for breeding sites within the AAT.
Globally, the population trend appears variable at between a 17% increase to a 7% decline in the global population over three generations (BirdLife International 2018g). There were an estimated 46,100 breeding pairs in 2021 (ACAP 2022).
Habitat critical to survival of species
Heard Island and McDonald Islands, and Macquarie Island, are subject to management plans that protect these listed world heritage sites and adjacent marine reserves (Parks and Wildlife Service 2006, Commonwealth of Australia 2014). The Antarctic breeding sites are all located in designated Antarctic Specially Protected Areas and subject to management plans (ATCM 2013, 2015, 2016). All breeding populations of Southern Giant Petrel in Australia’s jurisdiction are likely to be important for the long-term persistence of the species within Australia.
The species occupies 193 breeding sites in Antarctica (Adélie Land, Antarctic Peninsula, South Orkney Islands, South Shetland Islands) and on island groups of Argentina (Isla Arce, Isla de los Estados, Isla Gran Robredo, Isla Observatorio), Australia (AAT, Heard Island, McDonald Islands, Macquarie Island), Chile (Isla Noir, Islas Diego Ramirez), France (Crozet Islands, Kerguelen Islands), South Africa (Prince Edward Islands), United Kingdom (Gough Island), and other (Falkland Islands/as Malvinas, South Georgia/Islas Georgias del Sur, South Sandwich Islands/Islas Sandwich del Sur), with the largest population at the Falkland Islands/Islas Malvinas (ACAP 2012s).
Threats
The risk matrix for the Southern Giant Petrel is provided at Table 22, with the threats in Australia’s jurisdiction are highlighted.
Table 22: Southern Giant Petrel (Macronectes giganteus) risk matrix.
Likelihood of occurrence | Consequences |
Not significant | Minor | Moderate | Major | Catastrophic |
Almost certain | | Marine pollution: marine plastics ingestion Fisheries interactions: pelagic longline, demersal longline, trawl | | | |
Likely | | | | | |
Possible | | Marine pollution: heavy metal contamination Geological processes: volcanic activity leading to nest abandonment Human disturbance: at breeding sites leading to nest abandonment | | | |
Unlikely | | | | | |
Rare or Unknown | | Disease: avian cholera, avian pox virus | | | |
Note: Threats occurring in Australia’s jurisdiction are highlighted in bold.
Figure 5: Modelled Australian distribution of Southern Giant Petrel (Macronectes giganteus).

Family: Diomedeidae
Taxonomy
Macronectes halli Mathews 1912 is accepted nomenclature for the Northern Giant Petrel. Originally Macronectes giganteus halli Mathews 1912. The Northern Giant Petrel was considered polytypic with Macronectes giganteus (Southern Giant Petrel) until Bourne & Warham (1966) proposed recognition at the specific level based on morphology and demographic differences, a view also supported by genetic differences (Nunn & Stanley (1998), despite some hybridisation (Hunter 1983). ACAP has concluded on advice from its Taxonomy Working Group that available data warrant recognition of the Northern Giant Petrel at the specific level with the nomenclature widely accepted (Brooke et al. 2007, ACAP 2012n).
Current status of taxon
Environment Protection and Biodiversity Conservation Act 1999 (Commonwealth): Vulnerable
Biodiversity Conservation Act 2016 (Western Australia): not listed
National Parks and Wildlife Act 1972 (South Australia): not listed
Flora and Fauna Guarantee Act 1988 (Victoria): Endangered
Threatened Species Protection Act 1995 (Tasmania): Rare
Biodiversity Conservation Act 2016 (New South Wales): Vulnerable
Nature Conservation Act 1992 (Queensland): Vulnerable
IUCN Red list of Threatened Species: Least Concern
Action Plan for Australian Birds 2020: breeding population Least Concern, population visiting Australia Least Concern
Species description
A large petrel, the Northern Giant Petrel is approximately 87 cm in length, 2.9-5.3 kg in weight, with a wing length of 48-57 cm, and bill length of 85-111 mm (ACAP 2015, Menkhorst et al. 2017). Tubenosed; separate nostrils on a bulbous, pink plated bill with pale reddish tip. Combination of grey-brown and white plumage, dark head with light eye, dark body with grey-brown upper wings, and dark underwings (Onley & Scofield 2007, BirdLife International 2017).
Life history
Breeding locality | Jurisdiction |
Macquarie Island | Australia |
Crozet Islands, Kerguelen Islands | France |
Antipodes Islands, Auckland Islands, Chatham Islands, Campbell Island | New Zealand |
Prince Edward Islands | South Africa |
South Georgia/Islas Georgias del Sur | Other |
There are 50 breeding sites for the Northern Giant Petrel that occur on island groups of Australia (Macquarie Island), France (Crozet Islands, Kerguelen Islands), New Zealand (Antipodes Islands, Auckland Islands, Chatham Islands, Campbell Island) South Africa (Prince Edward Islands), and other (South Georgia/Islas Georgias del Sur) (ACAP 2012n). The majority of the Northern Giant Petrel population breeds annually, however a significant proportion (15-40%) of non-breeding is evident each year during ‘sabbatical’ periods that average 2.7 years (Bourne & Warham 1966, Voisin 1988). Adults arrive at colonies in August with females laying a single egg in August to early October that hatches after incubation period of about 9 weeks from October to early December with fledging of chicks occurring after approximately 4 months from late January to late March (Patterson et al. 2008, DPIPWE 2021a). Mean annual breeding success varies by location, with breeding success estimated at 62% on Macquarie Island (ACAP 2012n, DPIPWE 2021a). The Northern Giant Petrel has a wide circumpolar range across the southern oceans. Juveniles return to commence breeding when birds are 4-11 years of age (Voisin 1988). Generation length is estimated at 17.1 years (Bird et al. 2020).
Northern Giant Petrel are diurnal feeders at sea that also scavenge on land during the day and at night (Le Bohec et al. 2003). Birds take prey at sea by surface seizing, surface filtering, surface diving, and surface plunging up to 2 m in pursuit of prey (Harper 1987). They are aggressive opportunists scavenging prey from other smaller birds (Harper et al. 1985, Harper 1987). Females are smaller than males (80% by weight) with both sexes scavenging on seal and penguin carrion, and predating albatross chicks (González-Solis 2004, Dilley et al. 2013). During breeding periods, females also feed extensively on other marine food resources and show more pelagic habits than males (González-Solis 2004).
Northern Giant Petrel disperse widely throughout the southern oceans, mainly north of the Antarctic Convergence (Patterson & Hunter 1999, Patterson et al. 2008, Trebilco et al. 2008). At Macquarie Island most breeding birds forage within 100 km of the island (Trebilco et al. 2008). In winter, birds from Macquarie Island are found as far as southern Africa and South America and are frequently observed in southern Australian waters (Marchant & Higgins 1990, Reid et al. 2002, Trebilco et al. 2008).
Species distribution in Australia
Northern Giant Petrel nests on Macquarie Island (Figure 6). Tracking and banding studies indicate that dispersal is circumpolar, predominantly between 30-64°S and includes southern and sub-Antarctic Australia, however records at sea are subject to misidentification between the giant petrel species (Voisin 1988, BirdLife International 2004, ACAP 2012n). During the breeding cycle birds breeding on Macquarie Island generally forage southwards to the Antarctic ice edge or eastwards to South America (Trebilco et al. 2008).
The size of the breeding population of Northern Giant Petrel on Macquarie Island before human visitation is unknown. Harvesting of eggs and/or birds may have occurred during the 19th Century while the island was occupied by sealers (Cumpston 1968, Townrow 1988). Northern Giant Petrel is subject to ongoing long-term monitoring on Macquarie Island. An island-wide census of the breeding population on Macquarie Island was last conducted in 2013/14 with 1490 breeding pairs recorded, with the population currently monitored at a representative study site with 337 (248-391) annual breeding pairs recorded in 2019/20, and long-term breeding success estimated at 62% (DPIPWE 2021a).
Population estimates and trends
The breeding population of Northern Giant Petrel on Macquarie Island is considered uncertain based on a TRIM analysis (Pannekoek & van Strien 2006) over the past 10 years (DPIPWE 2021a), which is attributed to the impact of secondary poisoning in 2011 during work to eradicate pest species from the island (Parks & Wildlife Service 2014, DPIPWE 2021a).
Globally, the population appears to be increasing (BirdLife International 2017). There were an estimated 11,550 breeding pairs in 2021 (ACAP 2022).
Habitat critical to survival of species
Macquarie Island is subject to a management plan that protects this listed world heritage site and adjacent marine reserves (Parks and Wildlife Service 2006). The breeding population of Northern Giant Petrel in Australia’s jurisdiction is likely to be important for the long-term persistence of the species within Australia. The species is limited to 50 breeding sites on island groups of Australia (Macquarie Island), France (Crozet Islands, Kerguelen Islands), New Zealand (Antipodes Islands, Auckland Islands, Chatham Islands, Campbell Island) South Africa (Prince Edward Islands), and other (South Georgia/Islas Georgias del Sur), with the largest population at South Georgia/Islas Georgias del Sur (ACAP 2012n).
Threats
The risk matrix for the Northern Giant Petrel is provided at Table 23, with the threats occurring in Australia’s jurisdiction highlighted.
Table 23: Northern Giant Petrel (Macronectes halli) risk matrix.
Likelihood of occurrence | Consequences |
Not significant | Minor | Moderate | Major | Catastrophic |
Almost certain | | Marine pollution: marine plastics ingestion Fisheries interactions: pelagic longline, demersal longline, trawl | | | |
Likely | | Climate variability and change: habitat damage from severe storms affecting Macquarie Island | | | |
Possible | | Marine pollution: heavy metals and pesticide contamination Human disturbance: at breeding sites leading to nest abandonment | | | |
Unlikely | | | | | |
Rare or Unknown | | Climate variability and change: heat stress and degradation of nesting habitat | | | |
Note: Threats occurring in Australia’s jurisdiction are highlighted in bold.
Figure 6: Modelled Australian distribution of Northern Giant Petrel (Macronectes halli).

Family: Diomedeidae
Taxonomy
Procellaria cinerea Gmelin 1789 is accepted nomenclature for the Grey Petrel. Originally Procellaria cinerea Gmelin 1789. The nomenclature for this monotypic species is widely accepted (ACAP 2012i).
Current status of taxon
Environment Protection and Biodiversity Conservation Act 1999 (Commonwealth): not listed
Biodiversity Conservation Act 2016 (Western Australia): not listed
National Parks and Wildlife Act 1972 (South Australia): not listed
Flora and Fauna Guarantee Act 1988 (Victoria): not listed
Threatened Species Protection Act 1995 (Tasmania): Endangered
Biodiversity Conservation Act 2016 (New South Wales): not listed
Nature Conservation Act 1992 (Queensland): species not recorded in Queensland
IUCN Red list of Threatened Species: Near Threatened
Action Plan for Australian Birds 2020: breeding population Near Threatened, population visiting Australia Near Threatened
Species description
A small Procellaria petrel, the Grey Petrel is approximately 50 cm in length, 0.9-1.1 kg in weight, with a wing length of 29-35 cm, and bill length of 44-50 mm (ACAP 2015, Menkhorst et al. 2017). Tubenosed; separate nostrils on a pale plated bill. Combination of grey and white plumage, upper body grey, with darker cap to head and tail, with white underparts except for grey underwings and tail (Onley & Scofield 2007, BirdLife International 2018k).
Life history
Breeding locality | Jurisdiction |
Macquarie Island | Australia |
Amsterdam Island, Crozet Islands, Kerguelen Islands | France |
Antipodes Islands, Campbell Islands | New Zealand |
Prince Edward Islands | South Africa |
Gough Island, Tristan da Cunha | United Kingdom |
There are 17 breeding sites for the Grey Petrel that occur on island groups of Australia (Macquarie Island), France (Amsterdam Island, Crozet Islands, Kerguelen Islands), New Zealand (Antipodes Islands, Campbell Island), South Africa (Prince Edward Islands), and United Kingdom (Gough Island, Tristan da Cunha) (ACAP 2012i). The Grey Petrel is an annual winter breeder when successful (Jouventin et al. 1985, Chastel 1995). The breeding biology is variable with adults arriving at colonies from early February with females laying a single egg from late March to early April that hatches after incubation period of 7-9 weeks from late May to early June, with fledging of chicks occurring after 4-5 months from August to early December (Jouventin et al. 1985, Zotier 1990). Mean annual breeding success varies by location from 13-45% at sites outside the Australia’s jurisdiction (ACAP 2012i), while breeding success at Macquarie Island has consistently been ≥75% since rodent and rabbit eradication in 2012-2014 (Bird et al. in review, DPIPWE 2021a).
The Grey Petrel has a wide circumpolar range across the southern oceans and subtropical South America. Juveniles return to commence breeding when birds are an average of seven years of age (Barbraud et al. 2009). Generation length is estimated at 16.9 years (Bird et al. 2020).
Grey Petrel mostly forage alone, but may be found in small groups (around a dozen birds) including around fishing vessels and cetaceans (Harper 1987). Birds take prey by surface seizing and deep plunge diving (Harper 1987). Grey Petrel forage during daylight and return to their burrows at night. Adult birds usually depart burrows before dawn and return at dusk, with a few birds return to breeding colonies during the day (Bell et al. 2013). Grey Petrel diet is comprised mostly of cephalopods and fish and crustaceans and includes offal and discards from fishing vessels (Ridoux 1994).
Grey petrels range widely throughout the southern oceans with foraging areas demonstrating sexual segregation. During the breeding season females forage further north than males, in waters north of the Subtropical Convergence, over 1400 km from breeding sites (Bartle 1990). Grey Petrel re-established a breeding population on Macquarie Island after an 80-year absence (Schultz et al. 2005). After the breeding season, birds disperse widely.
Species distribution in Australia
The Grey Petrel breeds at Macquarie Island. An indicative distribution map is not available for this non-threatened species. Tracking studies indicate that dispersal is predominantly circumpolar between 32-58°S, extending to 18°S on the west coast of South America, and including southern and sub-Antarctic Australia (Marchant & Higgins 1990, BirdLife International 2004, ACAP 2012i).
The size of the breeding population of Grey Petrel on Macquarie Island before human visitation is unknown with the current population based on recolonization after an absence of some 80 years (to 2000) (Schultz et al. 2005). The extirpation was believed to be due to predation of this burrowing petrel species by feral cat (Felis catus) (Jones 1980). Grey Petrel monitoring has recommenced on Macquarie Island with an island-wide census of the breeding population undertaken in 2018, however population demographics are not yet available (DPIPWE 2021a). The latest population estimate, derived from an island-wide survey in 2018, is 252 (95% CI 227-302) breeding pairs (Bird et al. 2021a, 2021b, DPIPWE 2021a).
Population estimates and trends
While there is no population trend information available for the species over three generations due to a lack of data, the global population is suspected to be moderately rapidly declining (BirdLife International 2018k). There were an estimated 86,900 breeding pairs in 2018, however this estimate did not including the population on the Prince Edward Islands (ACAP 2022).
Habitat critical to survival of species
Macquarie Island is subject to a management plan that protects this listed world heritage site and adjacent marine reserves (Parks and Wildlife Service 2006). The breeding population of Grey Petrel in Australia’s jurisdiction is likely to be important for the long-term persistence of the species within Australia. The species is limited to 17 breeding sites on island groups of Australia (Macquarie Island), France (Amsterdam Island, Crozet Islands, Kerguelen Islands), New Zealand (Antipodes Islands, Campbell Island), South Africa (Prince Edward Islands), and United Kingdom (Gough Island, Tristan da Cunha), with the largest population at Antipodes Island (ACAP 2012i).
Threats
The risk matrix for the Grey Petrel is provided at Table 24, with the threats occurring in Australia’s jurisdiction highlighted.
Table 24: Grey Petrel (Procellaria cinerea) risk matrix.
Likelihood of occurrence | Consequences |
Not significant | Minor | Moderate | Major | Catastrophic |
Almost certain | | | Fisheries interactions: pelagic longline, demersal longline Introduced pest species: predation by cats, black rats, house mice | | |
Likely | | | | | |
Possible | | Human disturbance: at breeding sites leading to trampling of burrows | | | |
Unlikely | | | | | |
Rare or Unknown | | Disease: avian cholera | | | |
Note: Threats occurring in Australia's jurisdiction are highlighted in bold.
Family: Diomedeidae
Taxonomy
Diomedea amsterdamensis Roux et al. 1983 is accepted nomenclature for the Amsterdam Albatross. There has been considerable debate about the taxonomy for the wandering-type albatrosses (ACAP 2012a). Originally Diomedea exulans Linnaeus 1758. A review undertaken by Roux et al. (1983) recommended raising to specific level, based on the species’ adult plumage patterns, morphology and breeding biology (ACAP 2012a).
Current status of taxon
Environment Protection and Biodiversity Conservation Act 1999 (Commonwealth): Endangered
Biodiversity Conservation Act 2016 (Western Australia): Critically Endangered
National Parks and Wildlife Act 1972 (South Australia): not listed
Flora and Fauna Guarantee Act 1988 (Victoria): not listed
Threatened Species Protection Act 1995 (Tasmania): not listed
Biodiversity Conservation Act 2016 (New South Wales): not listed
Nature Conservation Act 1992 (Queensland): species not recorded in Queensland
IUCN Red list of Threatened Species: Endangered
Action Plan for Australian Birds 2020: population visiting Australia Endangered
Species description
A large albatross, the Amsterdam Albatross is approximately 100-110 cm in length, 4.8-8.0 kg in weight, with a wing length of 62-68 cm, and bill length of 138-156 mm (ACAP 2015, Menkhorst et al. 2017). Tubenosed; separate nostrils on a large, pink plated bill with greenish tip, and distinctive dark cutting edge. Eye with white eyelid. Combination of white and brown plumage, with mostly dark brown upperparts, with variable white on neck and back, with face and underparts mostly white, except for brown wingtips, with variable brown breast band (Onley & Scofield 2007, BirdLife International 2018a). Very similar in plumage to Wandering Albatross.
Life history
Breeding locality | Jurisdiction |
Amsterdam Island | France |
Endemic to the Amsterdam Island (France) with one breeding site on the island. The Amsterdam Albatross is a biennial breeder, when successful. On average, each breeding pair produces one egg every 1.8 years and fledges a chick every 2.4 years (Jouventin et al. 1989). Adult birds begin arriving at Amsterdam Island in January. Females lay a single egg in late February/March that hatches after an incubation period of over 11 weeks in May. The chicks fledge in January/February after spending at least eight months in the nest (Jouventin et al. 1989). Mean annual breeding success was estimated at 72% (Weimerskirch et al. 1997). The offspring then range the oceans for 4-7 years before returning to the island. Individuals do not begin breeding until they are nine years of age. Generation length is estimated at 22.3 years (Bird et al. 2020).
Species distribution in Australia
Australia is within the foraging range of the Amsterdam Albatross (Figure 7). The pelagic range for this species is poorly known, because of the similar appearance to other albatross species, such as the Wandering Albatross. Most sightings have been of birds in the Indian Ocean, with tracking studies indicating that adults forage generally within 2000 km of Amsterdam Island during the breeding season, while juveniles disperse across the Indian Ocean including adjacent to southwest Western Australia and eastern Africa (BirdLife International 2004, ACAP 2012a).
Population estimates and trends
The current global population trend is increasing (BirdLife International 2018a). Although numbers have been increasing since the late 1990s, the species remains among the world’s rarest seabirds (BirdLife International 2018a). There were an estimated 50 breeding pairs in 2020 (ACAP 2022).
Habitat critical to survival of species
Species is limited to one breeding site on Amsterdam Island (France) in the southern Indian Ocean.
Threats
The risk matrix for the Amsterdam Albatross is provided at Table 25, with the threats occurring in Australia’s jurisdiction highlighted.
Table 25: Amsterdam Albatross (Diomedea amsterdamensis) risk matrix.
Likelihood of occurrence | Consequences |
Not significant | Minor | Moderate | Major | Catastrophic |
Almost certain | | Marine pollution: heavy metal contamination | Fisheries interactions: pelagic longline | | |
Likely | | | | | |
Possible | | | | Disease: avian cholera outbreak | |
Unlikely | | | | | |
Rare or Unknown | | Marine pollution: marine plastics ingestion | | Introduced pest species: predation by cats, ship rats, house mice | |
Note: Threats occurring in Australia’s jurisdiction are highlighted in bold.
Figure 7: Modelled Australian distribution of Amsterdam Albatross (Diomedea amsterdamensis).

Family: Diomedeidae
Taxonomy
Diomedea antipodensis Robertson and Warham 1992 nomenclature remains under debate for the Antipodean Albatross (Double 2006, ACAP 2012b). Originally Diomedea exulans Linnaeus 1758. There has been considerable debate about the taxonomy for the wandering-type albatrosses (ACAP 2012b). Diomedea antipodensis is listed under the EPBC Act at the specific level, with Diomedea antipodensis gibsoni (Gibson’s Albatross) listed as a subspecies. Robertson & Warham (1992) proposed the nomenclature Diomedea exulans antipodensis (and Diomedea exulans gibsoni). The species was recommended to be raised to specific level by Robertson & Nunn (1998) as Diomedea antipodensis and Diomedea gibsoni based on the species’ morphology and ecology, with ACAP concluding on advice from its Taxonomy Working Group that available data do not warrant the recognition of Antipodean and Gibson’s albatrosses as separate species (Double 2006, ACAP 2006).
Current status of taxon
Environment Protection and Biodiversity Conservation Act 1999 (Commonwealth): Vulnerable
Biodiversity Conservation Act 2016 (Western Australia): Endangered
National Parks and Wildlife Act 1972 (South Australia): not listed
Flora and Fauna Guarantee Act 1988 (Victoria): not listed
Threatened Species Protection Act 1995 (Tasmania): not listed
Biodiversity Conservation Act 2016 (New South Wales): Vulnerable
Nature Conservation Act 1992 (Queensland): Vulnerable
IUCN Red list of Threatened Species: Endangered
Action Plan for Australian Birds 2020: population visiting Australia Critically Endangered
Species description
A large albatross, the Antipodean Albatross is approximately 100-115 cm in length, 4.3-8.9 kg in weight, with a wing length of 60-70 cm, and bill length of 139-155 mm (ACAP 2015, Menkhorst et al. 2017). Tubenosed; separate nostrils on a large, pink plated bill with yellowish tip. Combination of white and brownish plumage with mostly dark blackish-brown upperparts with variable white on neck and back, with clean white face and mostly white underparts, except for dark wingtips, without brown breast band (Onley & Scofield 2007, BirdLife International 2018b). Very similar in plumage to Wandering Albatross.
Life history
Breeding locality | Jurisdiction |
Antipodes Islands, Auckland Islands, Campbell Island | New Zealand |
Endemic to New Zealand with six breeding sites on the sub-Antarctic Antipodes Islands, Auckland Islands and Campbell Island. The Antipodean Albatross is a biennial breeder, when successful. The breeding season varies by a few weeks between the islands, commencing on the Auckland Islands, then on the Antipodes Islands and later on Campbell Island. Adults begin arriving at colonies from December/January. Females lay a single egg in late December/February that hatches after an incubation period of over 11 weeks in March/April (Walker & Elliott 1999, 2005). The chicks fledge after nine months, in late December to early March in the following year (Robertson 1985, Marchant & Higgins 1990, Brooke 2004; Walker et al. 2017). Mean annual breeding success has been averaged at 63% annually (Walker and Elliott 1999, Walker & Elliott 2002). Antipodean Albatross disperse over the Tasman Sea and South Pacific Ocean (Marchant & Higgins 1990). Juveniles return to the breeding colonies when three years of age and individuals do not begin breeding until they are seven years of age. Generation length is estimated at 25.9 years (Bird et al. 2020).
Species distribution in Australia
Australia is within the foraging range of the Antipodean Albatross (Figure 8). Tracking studies indicate that the birds from the Antipodes Islands mostly forage in the Pacific Ocean east of New Zealand, and the range of non-breeding birds is larger than that of breeders (BirdLife International 2004, Walker & Elliott 2006, ACAP 2012b). Non-breeding males have the largest range, foraging off the coast of Chile, Antarctica and in the tropical South Pacific. Birds from the Auckland Islands use differing foraging strategies according to sex, with females tending to frequent the Tasman Sea in the vicinity of 40°S, while males either disperse westwards at lower latitudes or travel north-east towards the mid-Pacific Ocean (Elliot et al. 1995). Non-breeding male and female birds forage westwards to the south-eastern Indian Ocean, including southern and sub-Antarctic Australia (BirdLife International 2004, Walker & Elliott 2006, ACAP 2012b).
Population estimates and trends
Projected very rapid population decline of the global population over three generations (BirdLife International 2018b). A dramatic population crash occurred at the Antipodes Islands in 2005, and adult males from this breeding population have been declining annually at 6% and females at 12% (Walker & Elliott 2017). The decline appears in large part due to very high female mortality, in some years up to 20%, though reduced breeding success and increased recruitment age have exacerbated the problem (Elliot & Walker 2020). There were an estimated 7100 breeding pairs in 2020 (ACAP 2022).
Habitat critical to survival of species
Species is limited to six breeding sites in New Zealand on the Antipodes Islands, Auckland Islands and Campbell Island, with the largest colony on Antipodes Island (ACAP 2012b).
Threats
The risk matrix for the Antipodean Albatross is provided at Table 26, with the threats occurring in Australia’s jurisdiction highlighted.
Table 26: Antipodean Albatross (Diomedea antipodensis) risk matrix.
Likelihood of occurrence | Consequences |
Not significant | Minor | Moderate | Major | Catastrophic |
Almost certain | | Marine pollution: heavy metal contamination Marine pollution: marine plastics ingestion | Climate variability and change: habitat damage from severe storms, heat stress from higher temperatures, variation in Southern Oscillation Index | Fisheries interactions: pelagic longline | |
Likely | | Introduced pest species: predation by cats, pigs | | | |
Possible | | | | | |
Unlikely | | | | | |
Rare or Unknown | | | | | |
Note: Threats occurring in Australia's jurisdiction are highlighted in bold.
Figure 8: Modelled Australian distribution of Antipodean Albatross (Diomedea antipodensis).

Family: Diomedeidae
Taxonomy
Diomedea dabbenena Matthews 1929 is accepted nomenclature for the Tristan Albatross. Diomedea dabbenena was first recognised as a separate species by Mathews (1929), being sister taxon to Diomedea exulans Linnaeus 1758. There has been considerable debate about the taxonomy for the wandering-type albatrosses (ACAP 2012u). Taxonomic deliberations led to a proposed complex of subspecies including Diomedea exulans dabbenena with the Tristan Albatross later raised to the specific level, based on the species’ genetics, morphology and other character differences (Gales 1998, Robertson & Nunn 1998, Cuthbert et al. 2003a), with this nomenclature now widely accepted (ACAP 2012u).
Current status of taxon
Environment Protection and Biodiversity Conservation Act 1999 (Commonwealth): Endangered
Biodiversity Conservation Act 2016 (Western Australia): Critically Endangered
National Parks and Wildlife Act 1972 (South Australia): not listed
Flora and Fauna Guarantee Act 1988 (Victoria): not listed
Threatened Species Protection Act 1995 (Tasmania): not listed
Biodiversity Conservation Act 2016 (New South Wales): not listed
Nature Conservation Act 1992 (Queensland): species not recorded in Queensland
IUCN Red list of Threatened Species: Critically Endangered
Action Plan for Australian Birds 2020: population visiting Australia Critically Endangered
Species description
A large albatross, the Tristan Albatross is approximately 110 cm in length, 6.8-7.3 kg in weight, with a wing length of 60-65 cm, and bill length of 144-150 mm (ACAP 2015). Tubenosed; separate nostrils on a large, pink plated bill with yellowish tip. Combination of white and dark plumage with head, neck and body white, with black tip to tail, with mostly dark, mottled, blackish upper wings to dark at wingtips, and with mostly white underparts with black trailing edge to underwing (Onley & Scofield 2007, BirdLife International 2018c). Very similar in plumage to Wandering Albatross.
Life history
Breeding locality | Jurisdiction |
Tristan de Cunha | United Kingdom |
Endemic to Tristan de Cunha (United Kingdom) with two breeding sites on Gough Island, and Inaccessible Island (ACAP 2012u, McClelland et al. 2016). The Tristan Albatross is a biennial breeder, when successful. Adults begin arriving at colonies from November. Females lay a single egg in January that hatches after an incubation period of over two months in March/April. The chicks fledge after eight to nine months, in November to January in the following year (Cuthbert et al. 2004). Mean annual breeding success is declining, averaging 32% in 2007 (Wanless 2007), but reported as just over 9% in 2014 (Davis et al. 2015). The range of the Tristan Albatross extends over the southern Atlantic and Indian Oceans to southern Australia (Marchant & Higgins 1990). Juveniles return to the breeding colonies when 3-7 years of age and individuals begin breeding at a mean age of 10 years (Wanless 2007). Generation length is estimated at 21.2 years (Bird et al. 2020).
Species distribution in Australia
Australia is within the foraging range of the Tristan Albatross (Figure 9). Tracking studies indicate that during the breeding season the birds mostly forage in the southern Atlantic Ocean, but disperse more widely during non-breeding periods including across the Indian Ocean to south-western Australia (BirdLife International 2004, ACAP 2012u).
Population estimates and trends
Projected extremely rapid population decline of the global population over three generations (BirdLife International 2018c). There is presently very low adult survival (BirdLife International 2018c). There were an estimated 1450 breeding pairs in 2017 (ACAP 2022).
Habitat critical to survival of species
Species is limited to two islands in Tristan de Cunha (United Kingdom) on Gough Island and Inaccessible Island in the southern Atlantic Ocean. The global population is effectively limited to one breeding site, Gough Island, as only 2-3 pairs were reported as breeding on Inaccessible Island (ACAP 2012u, McClelland et al. 2016).
Threats
The risk matrix for the Tristan Albatross is provided at Table 27, with the threats occurring in Australia’s jurisdiction highlighted.
Table 27: Tristan Albatross (Diomedea dabbenena) risk matrix.
Likelihood of occurrence | Consequences |
Not significant | Minor | Moderate | Major | Catastrophic |
Almost certain | | Climate variability and change: nesting habitat degradation Marine pollution: heavy metal and pesticides contamination | Fisheries interactions: pelagic longline, trawl | Introduced pest species: predation by house mice | |
Likely | | | | | |
Possible | | | | | |
Unlikely | | | | | |
Rare or Unknown | | Marine pollution: marine plastics ingestion | | | |
Note: Threats occurring in Australia’s jurisdiction are highlighted in bold.
Figure 9: Modelled Australian distribution of Tristan Albatross (Diomedea dabbenena).

Family: Diomedeidae
Taxonomy
Diomedea epomophora Lesson 1825 is accepted nomenclature for the Southern Royal Albatross. Originally Diomedea epomophora Lesson 1825. Considered polytypic with Diomedea sanfordi (Northern Royal Albatross) until raised to specific level by Robertson & Nunn (1998) based on morphological differences between Diomedea epomophora and Diomedea sanfordi, with this nomenclature widely accepted (ACAP 2012t).
Current status of taxon
Environment Protection and Biodiversity Conservation Act 1999 (Commonwealth): Vulnerable
Biodiversity Conservation Act 2016 (Western Australia): Vulnerable
National Parks and Wildlife Act 1972 (South Australia): Vulnerable
Flora and Fauna Guarantee Act 1988 (Victoria): Critically Endangered
Threatened Species Protection Act 1995 (Tasmania): not listed
Biodiversity Conservation Act 2016 (New South Wales): not listed
Nature Conservation Act 1992 (Queensland): Least Concern
IUCN Red list of Threatened Species: Vulnerable
Action Plan for Australian Birds 2020: population visiting Australia Least Concern
Species description
A large albatross, the Southern Royal Albatross is approximately 107-122 cm in length, 6.6-9.5 kg in weight, with a wing length of 65-75 cm, and bill length of 166-190 mm (ACAP 2015, Menkhorst et al. 2017). Tubenosed; separate nostrils on a large, pink plated bill with black cutting edge on upper mandible. Combination of white and dark plumage with white head and back, white black-brown tipped tail, black upper wings with white extending back from leading edge of inner wings in a triangle, and with mostly white underparts with black trailing edge to underwing and black wingtips (Onley & Scofield 2007, BirdLife International 2018d).
Life history
Breeding locality | Jurisdiction |
Auckland Islands, Campbell Island | New Zealand |
Endemic to New Zealand with four breeding sites in the sub-Antarctic Auckland Islands, and Campbell Island (ACAP 2012t). The Southern Royal Albatross is a biennial breeder, when successful. Adults begin arriving at colonies from October. Females lay a single egg from late November to late December that hatches after an incubation period of 10-12 weeks in early February to early March (Waugh et al. 1997). The chicks fledge after seven to eight months, in early October to early November (Marchant & Higgins 1990). Mean annual breeding success varies between 62-77% annually (Waugh et al. 1997, Moore et al. 1997, Childerhouse et al. 2003). The Southern Royal Albatross has a circumpolar range in the higher latitudes of the southern hemisphere (30-55°S) (BirdLife International 2004, 2018d). Juveniles return to breeding colonies when 3-7 years of age, with individuals beginning breeding when 6-12 years of age (Marchant & Higgins 1990, Childerhouse et al. 2003, Wanless 2007). Generation length is estimated at 27.9 years (Bird et al. 2020).
Species distribution in Australia
Australia is within the foraging range of the Southern Royal Albatross (Figure 10). Tracking studies indicate that during the breeding season the birds mostly forage in New Zealand waters, but dispersal is circumpolar during non-breeding periods, including southern, sub-Antarctic Australia, and the AAT (BirdLife International 2004, ACAP 2012t).
Population estimates and trends
The global population is considered stable or possibly increasing (Moore et al. 1997, BirdLife International 2018d. There were an estimated 7900 breeding pairs in 2018 (ACAP 2022).
Habitat critical to survival of species
Species is limited to four breeding sites in New Zealand, on three of the Auckland Islands and Campbell Island in the south-western Pacific Ocean, with the global population predominantly found on Campbell Island (ACAP 2012t).
Threats
The risk matrix for the Southern Royal Albatross is provided at Table 28, with the threats occurring in Australia’s jurisdiction highlighted.
Table 28: Southern Royal Albatross (Diomedea epomophora) risk matrix.
Likelihood of occurrence | Consequences |
Not significant | Minor | Moderate | Major | Catastrophic |
Almost certain | Dependence on fisheries discards | Marine pollution: heavy metal contamination Marine pollution: marine plastics ingestion | Fisheries interactions: pelagic longline, demersal longline, trawl, ingestion of discarded hooks | | |
Likely | | | | | |
Possible | | Introduced pest species: predation by cats, habitat degradation by pigs, and spread of Dracophyllum shrub | | | |
Unlikely | | | | | |
Rare or Unknown | | Human disturbance: at breeding sites leading to nest abandonment | | | |
Note: Threats occurring in Australia’s jurisdiction are highlighted in bold.
Figure 10: Modelled Australian distribution of Southern Royal Albatross (Diomedea epomophora).

Family: Diomedeidae
Taxonomy
Diomedea sanfordi Murphy 1917 is accepted nomenclature for the Northern Royal Albatross. Originally one of two subspecies, but raised to specific level by Robertson & Nunn (1998) based on morphological differences between Diomedea sanfordi (Northern Royal Albatross) and Diomedea epomophora (Southern Royal Albatross) with this nomenclature widely accepted (ACAP 2012o).
Current status of taxon
Environment Protection and Biodiversity Conservation Act 1999 (Commonwealth): Endangered
Biodiversity Conservation Act 2016 (Western Australia): Endangered
National Parks and Wildlife Act 1972 (South Australia): Endangered
Flora and Fauna Guarantee Act 1988 (Victoria): not listed
Threatened Species Protection Act 1995 (Tasmania): not listed
Biodiversity Conservation Act 2016 (New South Wales): not listed
Nature Conservation Act 1992 (Queensland): species not recorded in Queensland
IUCN Red list of Threatened Species: Endangered
Action Plan for Australian Birds 2020: population visiting Australia Endangered
Species description
A large albatross, the Northern Royal Albatross is approximately 115 cm in length, 6.5-6.8 kg in weight, with a wing length of 61-67 cm, and bill length of 154-172 mm (ACAP 2015, Menkhorst et al. 2017). Tubenosed; separate nostrils on a large, pink plated bill with black cutting edge on upper mandible. Combination of white and dark plumage with white head and back, white potentially black tipped tail, all black upper wings, and with mostly white underparts with black trailing edge to underwing and black wingtips (Onley & Scofield 2007, BirdLife International 2018f).
Life history
Breeding locality | Jurisdiction |
Auckland Islands, Chatham Islands, South Island of New Zealand (at Taiaroa Head) | New Zealand |
Endemic to New Zealand with five breeding sites on the sub-Antarctic Chatham Islands and Auckland Islands, and at Taiaroa Head on the South Island of New Zealand (ACAP 2012t). The Northern Royal Albatross is a biennial breeder, when successful. Adults begin arriving at colonies from August to mid-November. Females lay a single egg in late October to early November that hatches after an incubation period of over 11 weeks in late January to early February, with the chicks mostly fledging in September/October after spending at least eight months in the nest (Tickell 2000). The Northern Royal Albatross has a circumpolar distribution in the higher latitudes of the southern hemisphere (generally 36-52°S) (BirdLife International 2004, 2018f). Mean annual breeding success at Taiaroa Head was estimated as 31% (Westerskov 1963 in Marchant & Higgins 1990). Juveniles return to breeding colonies when at least three years of age, with individuals usually beginning breeding when eight years of age (Robertson 1993, 1998). Generation length is estimated at 26.0 years (Bird et al. 2020).
Species distribution in Australia
Australia is within the foraging range of the Northern Royal Albatross (Figure 11). Tracking studies indicate that during the breeding season the birds mostly forage in New Zealand waters, but dispersal is circumpolar during non-breeding periods, including southern and sub-Antarctic Australia, and AAT (BirdLife International 2004, ACAP 2012o).
Population estimates and trends
The global population is projected to be decreasing over three generations with uncertainty about the extent of the projected decline (BirdLife International 2018f). There were an estimated 4080 breeding pairs in 2018 (ACAP 2022).
Habitat critical to survival of species
Species is limited to five breeding sites in New Zealand, on the Auckland Islands, Chatham Islands, Campbell Island, and Taiaroa Head on the South Island of New Zealand in the south-western Pacific Ocean, with the global population predominantly found on the Chatham Islands (ACAP 2012o).
Threats
The risk matrix for the Northern Royal Albatross is provided at Table 29, with the threats occurring in Australia’s jurisdiction highlighted.
Table 29: Northern Royal Albatross (Diomedea sanfordi) risk matrix.
Likelihood of occurrence | Consequences |
Not significant | Minor | Moderate | Major | Catastrophic |
Almost certain | | Climate variability and change: habitat damage from severe storms, heat stress and degradation of nesting habitat from higher temperatures Marine pollution: heavy metal contamination Marine pollution: marine plastics ingestion | Fisheries interactions: pelagic longline, demersal longline, trawl | | |
Likely | | | | | |
Possible | | Introduced pest species: predation by brown rats, stoats | | | |
Unlikely | | | | | |
Rare or Unknown | | | | | |
Note: Threats occurring in Australia’s jurisdiction are highlighted in bold.
Figure 11: Modelled Australian distribution of Northern Royal Albatross (Diomedea sanfordi).

Family: Diomedeidae
Taxonomy
Phoebetria fusca (Hilsenberg 1822) is accepted nomenclature for the Sooty Albatross. Originally Diomedea fusca Hilsenberg 1822. The genus Phoebetria was introduced by Reichenbach (1852) and a review by Nichols & Murphy (1914) included the Sooty Albatross within the genus as Phoebetria fusca. Genetic analyses support this nomenclature (Robertson & Nunn 1998, Nunn et al. 1996) with the nomenclature widely accepted (ACAP 2012r).
Current status of taxon
Environment Protection and Biodiversity Conservation Act 1999 (Commonwealth): Vulnerable
Biodiversity Conservation Act 2016 (Western Australia): Endangered
National Parks and Wildlife Act 1972 (South Australia): Endangered
Flora and Fauna Guarantee Act 1988 (Victoria): Critically Endangered
Threatened Species Protection Act 1995 (Tasmania): Rare
Biodiversity Conservation Act 2016 (New South Wales): Vulnerable
Nature Conservation Act 1992 (Queensland): Vulnerable
IUCN Red list of Threatened Species: Endangered
Action Plan for Australian Birds 2020: population visiting Australia Near Threatened
Species description
A small-medium albatross, the Sooty Albatross is approximately 84-89 cm in length, 2.3-2.7 kg in weight, with a wing length of 49-54 cm, and bill length of 101-117 mm (ACAP 2015, Menkhorst et al. 2017). Tubenosed; separate nostrils on a large, black plated bill, with yellow to orange bill stripe on lower mandible. Generally, dark brownish-black plumage, except for partial white ring to eye and lighter base to the primaries, with slender wings and wedge-pointed tail (Onley & Scofield 2007, BirdLife International 2018h).
Life history
Breeding locality | Jurisdiction |
Amsterdam Island, Crozet Islands, Kerguelen Islands, Saint Paul Island | France |
Marion Island, Prince Edward Island | South Africa |
Tristan da Cunha | United Kingdom |
There are 15 breeding sites for the Sooty Albatross that occur on island groups of France (Amsterdam Island, Crozet Islands, Kerguelen Islands, Saint Paul Island), South Africa (Marion Island, Prince Edward Island), and Tristan de Cunha (United Kingdom) (ACAP 2012r). The Sooty Albatross is predominantly a biennial breeder, when successful (Ryan 2007). Adults mostly arrive at colonies in August/September. Females lay a single egg from mid-September to late October that hatches after incubation period of 9-10 weeks in mid-December (Berruti 1979, Weimerskirch et al. 1986). Fledging of chicks occurs after approximately 4-6 months in May-June (Berruti 1979, Weimerskirch et al. 1986, 1987). Annual breeding success estimated for one site varied significantly, averaging 58% (range 10-85%) (Weimerskirch & Jouventin 1998). The Sooty Albatross disperses widely in the southern Atlantic Ocean and southern Indian Ocean. Juveniles return to breeding colonies when eight years old, and begin beginning breeding at an average age of 12 years of age (Weimerskirch et al. 1987). Generation length is estimated at 21.1 years (Bird et al. 2020).
Species distribution in Australia
Australia is within the foraging range of the Sooty Albatross (Figure 12). Tracking studies indicate that dispersal is generally between 30-60°S in the southern Atlantic Ocean and southern Indian Ocean, but may extend to between 10-65°S, and includes southern and sub-Antarctic Australia (Tickell 2000, BirdLife International 2004, ACAP 2012r).
Population estimates and trends
The global population is experiencing a very rapid decline over three generations, but there is uncertainty about the trend due to variability between population counts (BirdLife International 2018h). There were an estimated 12,000 breeding pairs in 2021 (ACAP 2022).
Habitat critical to survival of species
Species is limited to 15 breeding sites on island groups of France (Amsterdam Island, Crozet Islands, Kerguelen Islands, Saint Paul Island), and South Africa (Marion Island, Prince Edward Island), and Tristan de Cunha (United Kingdom), with the largest population on Gough Island at Tristan da Cunha (ACAP 2012r).
Threats
The risk matrix for the Sooty Albatross is provided at Table 30, with the threats occurring in Australia’s jurisdiction highlighted.
Table 30: Sooty Albatross (Phoebetria fusca) risk matrix.
Likelihood of occurrence | Consequences |
Not significant | Minor | Moderate | Major | Catastrophic |
Almost certain | | Marine pollution: heavy metal contamination Marine pollution: marine plastics ingestion | Fisheries interactions: pelagic longline Introduced pest species: predation by cats, ship rats, house mice | | |
Likely | | | Disease: avian cholera, Erysipelas outbreaks | | |
Possible | | | | | |
Unlikely | | | | | |
Rare or Unknown | | | | | |
Note: Threats occurring in Australia’s jurisdiction are highlighted in bold.
Figure 12: Modelled Australian distribution of Sooty Albatross (Phoebetria fusca).
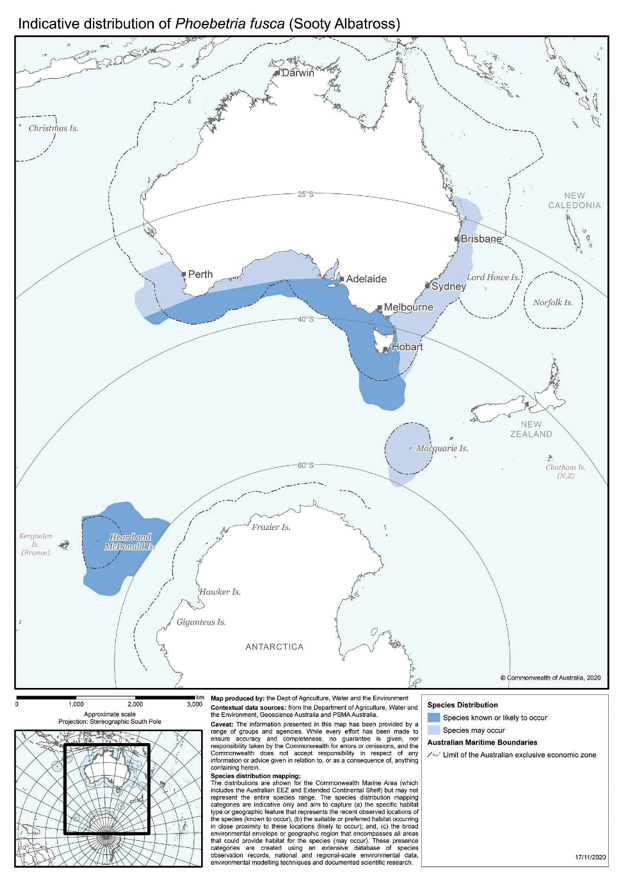
Family: Diomedeidae
Taxonomy
Thalassarche bulleri (Rothschild 1893) nomenclature for Buller’s Albatross remains under debate (Double 2006, ACAP 2012f). There has been significant taxonomic debate about the classification of Diomedeidae including, but not limited to, the introduction of the genus Thalassarche by Reichenbach (1852). Originally Diomedea bulleri Rothschild 1893 and Diomedea platei Reichenow 1898, with the latter later considered by Murphy (1936) as a juvenile plumage phase of the former. Buller’s Albatross was included in the resurrected genus Thalassarche (Reichenbach 1852) at the specific level based on genetic analyses (Nunn et al. 1996). Thalassarche bulleri is listed under the EPBC Act at the specific level, with Thalassarche bulleri platei (Northern Buller’s Albatross) listed as a subspecies. Robertson & Nunn (1998) suggested Thalassarche bulleri and Thalassarche platei as distinct terminal taxa based on morphological differences. ACAP has concluded on advice from its Taxonomy Working Group that available data did not warrant the recognition of Buller’s and Pacific albatrosses as separate species (Double 2006, ACAP 2006).
Current status of taxon
Environment Protection and Biodiversity Conservation Act 1999 (Commonwealth): Vulnerable
Biodiversity Conservation Act 2016 (Western Australia): not listed
National Parks and Wildlife Act 1972 (South Australia): Vulnerable
Flora and Fauna Guarantee Act 1988 (Victoria): Endangered
Threatened Species Protection Act 1995 (Tasmania): not listed
Biodiversity Conservation Act 2016 (New South Wales): not listed
Nature Conservation Act 1992 (Queensland): Vulnerable
IUCN Red list of Threatened Species: Near Threatened
Action Plan for Australian Birds 2020: population visiting Australia Vulnerable
Species description
A small-medium albatross, Buller’s Albatross is approximately 76-81 cm in length, 2.0-3.3 kg in weight, with a wing length of 47-55 cm, and bill length of 76-81 mm (ACAP 2015, Menkhorst et al. 2017). Tubenosed; separate nostrils on a large, mostly black plated bill with yellow upper and lower ridges, with yellow ridge broadening at base of bill. Combination of grey and white plumage, with white-capped, grey head and dark eye patch, white body with a dark back, upper wings, leading edge of underwings and tip of tail (Onley & Scofield 2007, BirdLife International 2018n).
Life history
Breeding locality | Jurisdiction |
Chatham Islands, Solander Islands, Snares Islands, Three Kings Islands | New Zealand |
Endemic to New Zealand with 10 breeding sites on the Chatham Islands, Solander Islands, Snares Islands, and Three Kings Islands (ACAP 2012f). Buller’s Albatross is typically an annual breeder with the breeding cycle at the Snares and Solander Islands commencing about three months later than at the Chatham and Three Kings Islands. Adults arrive at the Snares and Solander Islands in mid-December. Females lay a single egg in January/February that hatches after incubation period of around nine weeks in mid-March to April (Sagar & Warham 1998). Fledging of chicks occurs after approximately five and a half months in August to October (Warham & Bennington 1983, Sagar & Warham 1998). At the Chatham Islands, eggs are laid in November, hatch in January and chicks fledge in June (Robertson 1985, 1991). Mean annual breeding success varies by location from 51-71% (Sagar et al. 2002). Buller’s Albatross ranges across the southern Pacific Ocean and western seaboard of South America. Juveniles begin returning to colonies after at least three years, and commence breeding on average when 10-11 years of age (Francis & Sagar 2012). Generation length is estimated at 19.6 years (Bird et al. 2020).
Species distribution in Australia
Australia is within the foraging range of Buller’s Albatross (Figure 13). Tracking studies and at-sea studies indicate that dispersal from the breeding colonies is generally in the higher latitudes of the Pacific Ocean, but may extend northwards along the west coast of South America to the equator, and includes south-eastern Australia (Stahl et al. 1998, BirdLife International 2004, ACAP 2012b).
Population estimates and trends
The global population is considered stable and there are no projections of decline over three generations (BirdLife International 2018n). There were an estimated 33,200 breeding pairs in 2019 (ACAP 2022).
Habitat critical to survival of species
The species is limited to 10 breeding sites in New Zealand on the Chatham Islands, Solander Islands, Snares Islands, and Three Kings Islands, with the largest population on the Forty Fours in the Chatham Islands (ACAP 2012b).
Threats
The risk matrix for Buller’s Albatross is provided at Table 31, with the threats occurring in Australia’s jurisdiction highlighted.
Table 31: Buller’s Albatross (Thalassarche bulleri) risk matrix.
Likelihood of occurrence | Consequences |
Not significant | Minor | Moderate | Major | Catastrophic |
Almost certain | | Competition with native species: habitat damage by fur seals, predation of chicks by Weka (Gallirallus australis) Marine pollution: marine plastics ingestion | Fisheries interactions: pelagic longline, demersal longline, trawl, artisanal fishing | | |
Likely | | | Climate variability and change: habitat damage from severe storms | | |
Possible | | | | | |
Unlikely | | | | | |
Rare or Unknown | | | | | |
Note: Threats occurring in Australia's jurisdiction are highlighted in bold.
Figure 13: Modelled Australian distribution of Buller’s Albatross (Thalassarche bulleri).

Family: Diomedeidae
Taxonomy
Thalassarche carteri (Rothschild 1903) is accepted nomenclature for the Indian Yellow-nosed Albatross (Robertson 2002, ACAP 2012k). There has been significant taxonomic debate about the classification of Diomedeidae including, but not limited to, the introduction of the genus Thalassarche by Reichenbach (1852). Originally Thalassogeron carteri Rothschild 1903, within the genus Thalassogeron that had been introduced by Baird et al. (1884). Thalassogeron carteri was considered distinct from Thalassogeron chlororhynchos Gmelin 1789, the nomenclature for the Atlantic Yellow-nosed Albatross at that time (Rothschild 1903). The Indian Yellow-nosed Albatross was included in the resurrected genus Thalassarche (Reichenbach 1852) at the specific level as Thalassarche carteri based on genetic analyses (Nunn et al. 1996, Robertson & Nunn 1998). ACAP has concluded on advice from its Taxonomy Working Group that available data warrant recognition of Indian Yellow-nosed Albatross at the specific level with the nomenclature generally accepted (Brooke et al. 2007, ACAP 2012k).
Current status of taxon
Environment Protection and Biodiversity Conservation Act 1999 (Commonwealth): Vulnerable
Biodiversity Conservation Act 2016 (Western Australia): Endangered
National Parks and Wildlife Act 1972 (South Australia): Endangered
Flora and Fauna Guarantee Act 1988 (Victoria): Endangered
Threatened Species Protection Act 1995 (Tasmania): not listed
Biodiversity Conservation Act 2016 (New South Wales): not listed
Nature Conservation Act 1992 (Queensland): Vulnerable
IUCN Red list of Threatened Species: Endangered
Action Plan for Australian Birds 2020: population visiting Australia Endangered
Species description
A small albatross, the Indian Yellow-nosed Albatross is approximately 75 cm in length, 2.5-2.9 kg in weight, with a wing length of 46-50 cm, and bill length of 111-124 mm (ACAP 2015, Menkhorst et al. 2017). Tubenosed; separate nostrils on a large, mostly black plated bill with yellow upper ridge that is generally narrow at base of bill. Combination of dark and white plumage, with head and rump white, with small dark eye-patch, with dark back, upper wings and tail, and leading edge of underwings and wing tips (Onley & Scofield 2007, BirdLife International 2018o).
Life history
Breeding locality | Jurisdiction |
Amsterdam Island, Crozet Islands, Kerguelen Islands | France |
Prince Edward Island, Saint Paul Island | South Africa |
There are six breeding sites for the Indian Yellow-nosed Albatross that occur on island groups of France (Amsterdam Island, Crozet Islands, Kerguelen Islands), and South Africa (Prince Edward Island, Saint Paul Island) (ACAP 2012k). The Indian Yellow-nosed Albatross is an annual breeder, when successful. Adults arrive at colonies in August with females laying a single egg in September/ October that hatches after incubation period of 10 weeks in November/December (Jouventin et al. 1983). Mean annual breeding success varies from 14-24% at one breeding site, with data not available for other sites (ACAP 2012k). Fledging of chicks occurs after approximately four months in March/April (Jouventin et al. 1983, Weimerskirch et al. 1986). The Indian Yellow-nosed Albatross disperses in the higher latitudes of the Indian Ocean and southwest Pacific Ocean. Juveniles return to colonies to begin breeding when 8-9 years of age (ACAP 2012k). Generation length is estimated at 19.8 years (Bird et al. 2020).
Species distribution in Australia
Australia is within the foraging range of the Indian Yellow-nosed Albatross (Figure 14). Tracking studies and at-sea records indicate that dispersal from the breeding colonies is generally in the higher latitudes of the Indian Ocean from southern Africa to southwest Australia, and occasionally extending into New Zealand waters in the southwest Pacific Ocean (Gales 1998, BirdLife International 2004, Pinaud & Weimerskirch 2007, ACAP 2012k).
Population estimates and trends
The global population is experiencing a very rapid decline over three generations (BirdLife International 2018o). There were an estimated 33,950 breeding pairs in 2016 (ACAP 2022).
Habitat critical to survival of species
The species is limited to six breeding sites on island groups of France (Amsterdam Island, Crozet Islands, Kerguelen Islands), and South Africa (Prince Edward Island, Saint Paul Island), with the largest population on Amsterdam Island (ACAP 2012k).
Threats
The risk matrix for the Indian Yellow-nosed Albatross is provided at Table 32, with the threats occurring in Australia’s jurisdiction highlighted.
Table 32: Indian Yellow-nosed Albatross (Thalassarche carteri) risk matrix.
Likelihood of occurrence | Consequences |
Not significant | Minor | Moderate | Major | Catastrophic |
Almost certain | | Climate variability and change: sea temperature rise Introduced pest species: predation by cats, ship rats | Fisheries interactions: pelagic longline | | |
Likely | | | Disease: avian cholera, Erysipelas outbreaks | | |
Possible | | | | | |
Unlikely | | | | | |
Rare or Unknown | | | | | |
Note: Threats occurring in Australia’s jurisdiction are highlighted in bold.
Figure 14: Modelled Australian distribution of Indian Yellow-nosed Albatross (Thalassarche carteri).

Family: Diomedeidae
Taxonomy
Thalassarche chlororhynchos (Gmelin 1789) is accepted nomenclature for the Atlantic Yellow-nosed Albatross (ACAP 2012c). There has been significant taxonomic debate about the classification of Diomedeidae including, but not limited to, the introduction of the genus Thalassarche by Reichenbach (1852). Originally Diomedea chlororhynchos Gmelin 1789. The Atlantic Yellow-nosed Albatross was included in the resurrected genus Thalassarche (Reichenbach 1852) at the specific level as Thalassarche chlororhynchos based on genetic analyses (Nunn et al. 1996, Robertson & Nunn 1998). ACAP has concluded on advice from its Taxonomy Working Group that available data warrant recognition of Atlantic Yellow-nosed Albatross at the specific level with the nomenclature generally accepted (Brooke et al. 2007, ACAP 2012c).
Current status of taxon
Environment Protection and Biodiversity Conservation Act 1999 (Commonwealth): not listed
Biodiversity Conservation Act 2016 (Western Australia): Vulnerable
National Parks and Wildlife Act 1972 (South Australia): Endangered
Flora and Fauna Guarantee Act 1988 (Victoria): not listed
Threatened Species Protection Act 1995 (Tasmania): not listed
Biodiversity Conservation Act 2016 (New South Wales): not listed
Nature Conservation Act 1992 (Queensland): species not recorded in Queensland
IUCN Red list of Threatened Species: Endangered
Action Plan for Australian Birds 2020: not listed
Species description
A small albatross, the Atlantic Yellow-nosed Albatross is approximately 75 cm in length, 1.8-2.8 kg in weight, with a wing length of 48-52 cm, and bill length of 107-122 mm (ACAP 2015, Menkhorst et al. 2017). Tubenosed; separate nostrils on a large, mostly black plated bill, with pink tip and yellow upper ridge that is generally rounded at base of bill. Combination of dark and white plumage, with head and rump white, with dark eye-patch and pale grey on cheek, with dark back, upper wings and tail, and leading edge of underwings and wing tips (Onley & Scofield 2007, BirdLife International 2018q).
Life history
Breeding locality | Jurisdiction |
Tristan da Cunha | United Kingdom |
Endemic to Tristan de Cunha (United Kingdom) with six breeding sites on Gough Island, Nightingale Island, Inaccessible Island, and Tristan da Cunha (ACAP 2012c). Although the Atlantic Yellow-nosed Albatross is generally an annual breeder, when successful, experienced breeding pairs only attempt breeding in two out of every three years (Cuthbert et al. 2003b). Adults arrive at colonies in late August/September with females laying a single egg in September/October that hatches after incubation period of just over nine weeks in late November to late December (Rowan 1951). Fledging of chicks occurs after approximately four months in April (Rowan 1951, Cuthbert et al. 2003b). Mean annual breeding success varies by location from 64-69% (ACAP 2012c).
The Atlantic-nosed Albatross disperses in the higher latitudes of the Atlantic Ocean and southwest Indian Ocean. Juveniles return to colonies when five years of age, and begin breeding when 6-13 years of age (Cuthbert 2003b). Generation length is estimated at 18.5 years (Bird et al. 2020).
Species distribution in Australia
Australia is within the foraging range of the Atlantic Yellow-nosed Albatross. An indicative distribution map is not available for this non-threatened species. Tracking studies and at-sea records indicate that dispersal from the breeding colonies is generally in the higher latitudes (25-50°S) of the Atlantic Ocean extending into the western Indian Ocean, with some birds reaching Australian and New Zealand waters (Robertson 1975, BirdLife International 2004, ACAP 2012c).
Population estimates and trends
The global population is experiencing a very rapid decline over three generations (Davies et al. 2015, BirdLife International 2018q). There were an estimated 33,650 breeding pairs in 2011 (ACAP 2022).
Habitat critical to survival of species
The species is limited to six breeding sites at Tristan de Cunha (United Kingdom) on Gough Island, Nightingale Island, Inaccessible Island, and Tristan da Cunha, with the largest population on Tristan da Cunha (ACAP 2012c).
Threats
The risk matrix for the Atlantic Yellow-nosed Albatross is provided at Table 33, with the threats occurring in Australia’s jurisdiction highlighted.
Table 33: Atlantic Yellow-nosed Albatross (Thalassarche chlororhynchos) risk matrix.
Likelihood of occurrence | Consequences |
Not significant | Minor | Moderate | Major | Catastrophic |
Almost certain | | Marine pollution: marine plastics ingestion | | Fisheries interactions: pelagic longline, trawl Introduced pest species: predation by house mice, ship rats | |
Likely | | | | | |
Possible | | Human disturbance: take of chicks and eggs for food at nesting sites | | | |
Unlikely | | | | | |
Rare or Unknown | | Disease: vector unknown | | | |
Note: Threats occurring in Australia’s jurisdiction are highlighted in bold.
Family: Diomedeidae
Taxonomy
Thalassarche eremita Murphy 1930 is generally accepted nomenclature for the Chatham Albatross (ACAP 2012h). There has been significant taxonomic debate about the classification of Diomedeidae including, but not limited to, the introduction of the genus Thalassarche by Reichenbach (1852). Originally Thalassarche cauta eremita Murphy 1930. The Chatham Albatross was considered polytypic until it was included in the resurrected genus Thalassarche (Reichenbach 1852) at the specific level as Thalassarche eremita based on morphology and demographic differences, and genetic analyses (Robertson & Nunn 1998). ACAP has concluded on advice from its Taxonomy Working Group that available data warrant recognition of Chatham Albatross at the specific level with the nomenclature generally accepted (Brooke et al. 2007, ACAP 2012h).
Current status of taxon
Environment Protection and Biodiversity Conservation Act 1999 (Commonwealth): Endangered
Biodiversity Conservation Act 2016 (Western Australia): not listed
National Parks and Wildlife Act 1972 (South Australia): not listed
Flora and Fauna Guarantee Act 1988 (Victoria): not listed
Threatened Species Protection Act 1995 (Tasmania): not listed
Biodiversity Conservation Act 2016 (New South Wales): not listed
Nature Conservation Act 1992 (Queensland): species not recorded in Queensland
IUCN Red list of Threatened Species: Vulnerable
Action Plan for Australian Birds 2020: population visiting Australia Least Concern
Species description
A medium albatross, the Chatham Albatross is approximately 70-85 cm in length, 3.1-4.7 kg in weight, with a wing length of 53-59 cm, and bill length of 113-130 mm (ACAP 2015, Menkhorst et al. 2017). Tubenosed; separate nostrils on a large, mostly yellow plated bill, with black tip to lower mandible. Combination of dark, grey and white plumage, with dark grey head and white body, with dark eye-patch, with black upper wings and back, and tail, and leading edge of underwings and wing tips (Onley & Scofield 2007, BirdLife International 2018s).
Life history
Breeding locality | Jurisdiction |
Chatham Islands | New Zealand |
Endemic to the Chatham Islands (New Zealand) with one breeding site on the Pyramid (ACAP 2012h). Chatham Albatross breed annually, when successful. Adults arrive at colonies in late August with females laying a single egg in September/October that hatches after incubation period of 9-10 weeks in November/December (Robertson et al. 2000). Fledging of chicks occurs after approximately four months in March/April (Robertson 1985). Data are not available for mean breeding success (ACAP 2012h). The Chatham Albatross disperses in the higher latitudes of the Pacific Ocean to South America. Juveniles return to colonies when at least four years of age, and begin breeding when seven years of age (Robertson et al. 2000). Generation length is estimated at 19.0 years (Bird et al. 2020).
Species distribution in Australia
Australia is within the foraging range of the Chatham Albatross (Figure 15). Tracking studies and at-sea records indicate that dispersal from the breeding colonies is generally in the higher latitudes of the Pacific Ocean to South America (BirdLife International 2004, Onley & Scofield 2007, ACAP 2012h), with some birds recorded in waters adjacent to south-eastern and sub-Antarctic Australia (Reid & James 1997), as well as southern Africa (Ryan 2002).
Population estimates and trends
The global population is considered stable, although this assessment requires confirmation, as there is no trend available for the species over three generations (Croxall & Gales 1998, BirdLife International 2018s). There were an estimated 5250 breeding pairs in 2017 (ACAP 2022).
Habitat critical to survival of species
The species is limited to one breeding site in the Chatham Islands (New Zealand) on the Pyramid (ACAP 2012h).
Threats
The risk matrix for the Chatham Albatross is provided at Table 34, with the threats occurring in Australia’s jurisdiction highlighted.
Table 34: Chatham Albatross (Thalassarche eremita) risk matrix.
Likelihood of occurrence | Consequences |
Not significant | Minor | Moderate | Major | Catastrophic |
Almost certain | | Competition with native species: habitat damage by fur seals Marine pollution: marine plastics ingestion | Fisheries interactions: pelagic longline, demersal longline, trawl | | |
Likely | | | Climate variability and change: habitat damage from severe storms, heat stress and degradation of nesting habitat from higher temperatures | | |
Possible | | Human disturbance: at nesting sites from take of chicks for food | | | |
Unlikely | | | | | |
Rare or Unknown | | | | | |
Note: Threats occurring in Australia’s jurisdiction are highlighted in bold.
Figure 15: Modelled Australian distribution of Chatham Albatross (Thalassarche eremita).
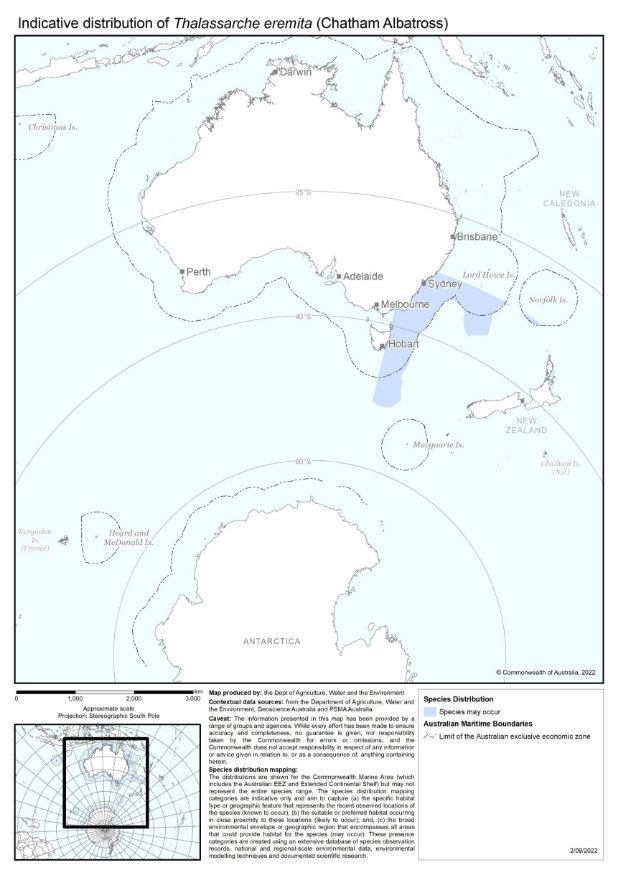
Family: Diomedeidae
Taxonomy
Thalassarche impavida Mathews 1912 is accepted nomenclature for the Campbell Albatross (ACAP 2012g). There has been significant taxonomic debate about the classification of Diomedeidae including, but not limited to, the introduction of the genus Thalassarche by Reichenbach (1852). Originally Thalassarche melanophris impavida Mathews 1912. The Campbell Albatross was considered polytypic until it was included in the resurrected genus Thalassarche (Reichenbach 1852) at the specific level as Thalassarche impavida based on genetic analyses (Robertson & Nunn 1998) with the nomenclature generally accepted (ACAP 2012g).
Current status of taxon
Environment Protection and Biodiversity Conservation Act 1999 (Commonwealth): Vulnerable
Biodiversity Conservation Act 2016 (Western Australia): Vulnerable
National Parks and Wildlife Act 1972 (South Australia): Vulnerable
Flora and Fauna Guarantee Act 1988 (Victoria): not listed
Threatened Species Protection Act 1995 (Tasmania): not listed
Biodiversity Conservation Act 2016 (New South Wales): not listed
Nature Conservation Act 1992 (Queensland): Least Concern
IUCN Red list of Threatened Species: Vulnerable
Action Plan for Australian Birds 2020: population visiting Australia Vulnerable
Species description
A small-medium albatross, the Campbell Albatross is approximately 80-95 cm in length, 2.2-3.8 kg in weight, with a wing length of 49-54 cm, and bill length of 105-118 mm (ACAP 2015, Menkhorst et al. 2017). Tubenosed; separate nostrils on a large, mostly orange plated bill, with reddish tip. Combination of black and white plumage, with white head and body, with black eyebrow and pale iris, with black upper wings and back, and tail, and extensive leading edge of underwings and wing tips (Onley & Scofield 2007, BirdLife International 2018t).
Life history
Breeding locality | Jurisdiction |
Campbell Island | New Zealand |
Endemic to Campbell Island (New Zealand) with two breeding sites on the island (ACAP 2012g). Campbell Albatross breeds annually, when successful. Adults arrive at colonies in August with females laying a single egg in late September to early October that hatches after incubation period of around 10 weeks in December (Marchant & Higgins 1990, Waugh et al. 1999). Fledging of chicks occurs after approximately four months in mid-April to early May (Waugh et al. 1999). Mean annual breeding success is 66% ± 12% (ACAP 2012g). The Campbell Albatross disperses in the higher latitudes of eastern Indian Ocean and western Pacific Ocean. Juveniles return to colonies when five years of age, and begin breeding when seven years of age (Waugh et al. 1999). Generation length is estimated at 21.6 years (Bird et al. 2020).
Species distribution in Australia
Australia is within the foraging range of the Campbell Albatross (Figure 16). Tracking studies and at-sea records indicate that dispersal from the breeding colonies is generally in the higher latitudes of the western Pacific Ocean and eastern Indian Ocean including waters adjacent to southern and eastern, and sub-Antarctic Australia, and the AAT (BirdLife International 2004, ACAP 2012g).
Population estimates and trends
The global population is considered to be increasing, although this assessment requires confirmation, as there is no trend available for the species over three generations (BirdLife International 2018t). There were an estimated 24,300 breeding pairs in 2020 (ACAP 2022).
Habitat critical to survival of species
The species is limited to two breeding sites on Campbell Island (New Zealand) (ACAP 2012g).
Threats
The risk matrix for the Campbell Albatross is provided at Table 35, with the threats occurring in Australia’s jurisdiction highlighted.
Table 35: Campbell Albatross (Thalassarche impavida) risk matrix.
Likelihood of occurrence | Consequences |
Not significant | Minor | Moderate | Major | Catastrophic |
Almost certain | | | Fisheries interactions: pelagic longline, demersal longline, trawl | | |
Likely | | | | | |
Possible | | | | | |
Unlikely | | | | | |
Rare or Unknown | | | | | |
Note: Threats occurring in Australia’s jurisdiction are highlighted in bold.
Figure 16: Modelled Australian distribution of Campbell Albatross (Thalassarche impavida).

Family: Diomedeidae
Taxonomy
Thalassarche salvini (Rothschild 1893) is accepted nomenclature for Salvin’s Albatross. There has been significant taxonomic debate about the classification of Diomedeidae including, but not limited to, the introduction of the genus Thalassarche by Reichenbach (1852). Originally Thalassogeron salvini Rothschild 1893. Salvin’s Albatross was considered polytypic until it was included in the resurrected genus Thalassarche (Reichenbach 1852) at the specific level as Thalassarche salvini based on morphology and demographic differences, and genetic analyses (Robertson & Nunn 1998). ACAP has concluded on advice from its Taxonomy Working Group that available data warrant recognition of Salvin’s Albatross at the specific level with the nomenclature generally accepted (Brooke et al. 2007, ACAP 2012p).
Current status of taxon
Environment Protection and Biodiversity Conservation Act 1999 (Commonwealth): Vulnerable
Biodiversity Conservation Act 2016 (Western Australia): Vulnerable
National Parks and Wildlife Act 1972 (South Australia): Vulnerable
Flora and Fauna Guarantee Act 1988 (Victoria): not listed
Threatened Species Protection Act 1995 (Tasmania): not listed
Biodiversity Conservation Act 2016 (New South Wales): not listed
Nature Conservation Act 1992 (Queensland): Least Concern
IUCN Red list of Threatened Species: Vulnerable
Action Plan for Australian Birds 2020: population visiting Australia Least Concern
Species description
A medium albatross, Salvin’s Albatross is approximately 90-100 cm in length, 3.3-4.9 kg in weight, with a wing length of 55-60 cm, and bill length of 109-121 mm (ACAP 2015, Menkhorst et al. 2017). Tubenosed; separate nostrils on a large, mostly grey bill with yellow upper ridge and black lower tip. Combination of black, grey and white plumage, with grey head and dark eye patch, with white body, with black upper wings, back and tail, and leading edge of underwings and wing tips, and a dark mark at base of leading edge of underwings (Onley & Scofield 2007, BirdLife International 2018v).
Life history
Breeding locality | Jurisdiction |
Bounty Islands, Snares Islands | New Zealand |
Endemic to New Zealand with 12 breeding sites on the Bounty Islands, and Snares Islands (ACAP 2012p). Salvin’s Albatross breeds annually, when successful. Adults arrive at colonies in August with females laying a single egg in late August/September. Although the incubation period is not known, hatching of chicks begins in late October (Sagar et al. 2004). Fledging of chicks occurs after approximately four months in March/April (Robertson & van Tets 1982, Miskelly et al. 2001). Mean annual breeding success unknown (ACAP 2012p). Salvin’s Albatross disperses in the higher latitudes of the Pacific Ocean. It is not known when juveniles first return to colonies or when they begin breeding (ACAP 2012p). Generation length is estimated at 19.2 years (Bird et al. 2020).
Species distribution in Australia
Australia is within the foraging range of Salvin's Albatross (Figure 17). At-sea records indicate that dispersal from the breeding colonies is generally in the higher latitudes of the Pacific Ocean including includes waters adjacent to southern and eastern, and sub-Antarctic Australia (BirdLife International 2004, ACAP 2012p).
Population estimates and trends
There is no global population trend information available for the species over three generations (BirdLife International 2018v). There were an estimated 26,450 breeding pairs in 2019 (ACAP 2022).
Habitat critical to survival of species
The species is limited to 12 breeding sites on the Bounty Islands and Snares Islands (New Zealand), with the largest population at the Bounty Islands (ACAP 2012p).
Threats
The risk matrix for Salvin's Albatross is provided at Table 36, with the threats occurring in Australia's jurisdiction highlighted.
Table 36: Salvin's Albatross (Thalassarche salvini) risk matrix.
Likelihood of occurrence | Consequences |
Not significant | Minor | Moderate | Major | Catastrophic |
Almost certain | | Competition with native species: habitat damage by fur seals | Fisheries interactions: pelagic longline, demersal longline, trawl | | |
Likely | | | | | |
Possible | | | | | |
Unlikely | | | | | |
Rare or Unknown | | | | | |
Note: Threats occurring in Australia’s jurisdiction are highlighted in bold.
Figure 17: Modelled Australian distribution of Salvin’s Albatross (Thalassarche salvini).

Family: Diomedeidae
Taxonomy
Thalassarche steadi Falla 1933 is generally accepted nomenclature for the White-capped Albatross. There has been significant taxonomic debate about the classification of Diomedeidae including, but not limited to, the introduction of the genus Thalassarche by Reichenbach (1852). Originally Thalassarche cauta steadi Falla 1933. The White-capped Albatross was considered polytypic until it was included in the resurrected genus Thalassarche (Reichenbach 1852) at the specific level as Thalassarche salvini based on morphology and demographic differences, and genetic analyses (Robertson & Nunn 1998). ACAP has concluded on advice from its Taxonomy Working Group that available data warrant recognition of the White-capped Albatross at the specific level with the nomenclature generally accepted (Double 2006, ACAP 2012x).
Current status of taxon
Environment Protection and Biodiversity Conservation Act 1999 (Commonwealth): Vulnerable
Biodiversity Conservation Act 2016 (Western Australia): Vulnerable
National Parks and Wildlife Act 1972 (South Australia): not listed
Flora and Fauna Guarantee Act 1988 (Victoria): not listed
Threatened Species Protection Act 1995 (Tasmania): not listed
Biodiversity Conservation Act 2016 (New South Wales): not listed
Nature Conservation Act 1992 (Queensland): Vulnerable
IUCN Red list of Threatened Species: Near Threatened
Action Plan for Australian Birds 2020: population visiting Australia Near Threatened
Species description
A medium albatross, the White-capped Albatross is approximately 90 cm in length, 3.3-4.4 kg in weight, with a wing length of 56-63 cm, and bill length of 126-141 mm (ACAP 2015, Menkhorst et al. 2017). Tubenosed; separate nostrils on a large, pale grey plated bill with yellow tip. Combination of black, grey and white plumage, white head with pale grey sides and dark eyebrow, white body with black upper wings, black margins and a dark tab intruding into the base of the underwings, and grey-black tail (Onley & Scofield 2007, BirdLife International 2018w).
Life history
Breeding locality | Jurisdiction |
Auckland Islands, Antipodes Islands | New Zealand |
Endemic to New Zealand with five breeding sites on the Auckland Islands, and Antipodes Islands (ACAP 2012x). The breeding cycle for the White-capped Albatross has not been fully described. The species exhibits an intermediate breeding strategy, with 63% breeding in one year, and 78% of failed breeders breeding in the next year (Francis 2012). Adults may be present at colonies year-round, with females laying a single egg in mid-November to mid-December that hatches after 9-10 weeks in mid-January to mid-February (Sagar 2017). Fledging of chicks occurs after approximately four months in June (Thompson et al. 2009). White-capped Albatross disperses in the higher latitudes of the southern hemisphere. It is not known when juveniles first return to colonies or when they begin breeding (ACAP 2012x). Generation length is estimated at 19.2 years (Bird et al. 2020).
Species distribution in Australia
Australia is within the foraging range of the White-capped Albatross (Figure 18). Tracking studies indicate that dispersal from the breeding colonies is generally in the higher latitudes of the southern hemisphere including waters adjacent to south-eastern Australia, particularly north-east of Tasmania (BirdLife International 2004, ACAP 2012x). At sea records are problematic due to similarities between, and overlapping range of White-capped Albatross and Shy Albatross (Abbott et al. 2006a).
Population estimates and trends
There is no global population trend information available for the species over three generations (BirdLife International 2018w). There were an estimated 62,900 breeding pairs in 2017 (ACAP 2022).
Habitat critical to survival of species
The species is limited to five breeding sites on the Auckland Islands, and Antipodes Islands (New Zealand), with the largest population on Disappointment Island in the Auckland Islands (ACAP 2012x).
Threats
The risk matrix for the White-capped Albatross is provided at Table 37, with the threats occurring in Australia’s jurisdiction highlighted.
Table 37: White-capped Albatross (Thalassarche steadi) risk matrix.
Likelihood of occurrence | Consequences |
Not significant | Minor | Moderate | Major | Catastrophic |
Almost certain | | Marine pollution: marine plastics ingestion | Fisheries interactions: pelagic longline, demersal longline, trawl | Introduced pest species: predation by cats, habitat degradation and predation by pigs | |
Likely | | | | | |
Possible | | | | | |
Unlikely | | | | | |
Rare or Unknown | | | | | |
Note: Threats occurring in Australia’s jurisdiction are highlighted in bold.
Figure 18: Modelled Australian distribution of White-capped Albatross (Thalassarche steadi).

Family: Diomedeidae
Taxonomy
Procellaria aequinoctialis Linnaeus 1758 is accepted nomenclature for the White-chinned Petrel. Originally Procellaria aequinoctialis Linnaeus 1758. The White-chinned Petrel was considered polytypic with Procellaria conspicillata (Spectacled Petrel) until Ryan (1998) proposed recognition at the specific level based on morphology and demographic differences, a view also supported by genetic differences (Techow et al 2009). ACAP has concluded on advice from its Taxonomy Working Group that available data warrant recognition of the White-chinned Petrel at the specific level with the nomenclature widely accepted (Brooke et al. 2007, ACAP 2012y).
Current status of taxon
Environment Protection and Biodiversity Conservation Act 1999 (Commonwealth): not listed
Biodiversity Conservation Act 2016 (Western Australia): Vulnerable
National Parks and Wildlife Act 1972 (South Australia): not listed
Flora and Fauna Guarantee Act 1988 (Victoria): not listed
Threatened Species Protection Act 1995 (Tasmania): not listed
Biodiversity Conservation Act 2016 (New South Wales): not listed
Nature Conservation Act 1992 (Queensland): Least Concern
IUCN Red list of Threatened Species: Vulnerable
Action Plan for Australian Birds 2020: population visiting Australia Least Concern
Species description
A small Procellaria petrel, the White-chinned Petrel is approximately 50-55 cm in length, 1.1-1.5 kg in weight, with a wing length of 42-47 cm, and bill length of 48-55 mm (ACAP 2015, Menkhorst et al. 2017). Tubenosed; separate nostrils on a yellow plated bill with black between nostrils and tip. Combination of sooty black and white plumage, with all sooty black plumage, except for a variable white chin and throat (Onley & Scofield 2007, BirdLife International 2018i).
Life history
Breeding locality | Jurisdiction |
Crozet Islands, Kerguelen Islands | France |
Antipodes Islands, Auckland Islands, Campbell Islands | New Zealand |
Prince Edward Islands | South Africa |
Falkland Islands/Islas Malvinas, South Georgia/Islas Georgias del Sur | Other |
There are 73 breeding sites for the White-chinned Petrel that occur on island groups of France (Crozet Islands, Kerguelen Islands), New Zealand (Antipodes Islands, Auckland Islands, Campbell Islands), South Africa (Prince Edward Islands), and other (Falkland Islands/Islas Malvinas, South Georgia/Islas Georgias del Sur) (ACAP 2012y). The White-chinned Petrel is an annual breeder when successful (Jouventin et al. 1985, Hall 1987). Adults arrive at colonies from September with females laying a single egg from mid-October to mid-November that hatches after incubation period of 8 weeks from mid-December to mid-January with fledging of chicks occurring after approximately 3 months in March/April (Jouventin et al. 1985, Hall 1987). Mean annual breeding success varies by location from 21-51% (ACAP 2012y). The White-chinned Petrel has a wide circumpolar range across the southern oceans and subtropical South America. Juveniles return to commence breeding when birds are 4-9 years of age (ACAP 2012y). Generation length is estimated at 18.6 years (Bird et al. 2020).
Species distribution in Australia
Australia is within the foraging range of the White-chinned Petrel. An indicative distribution map is not available for this non-threatened species. Tracking studies indicate that while dispersal is circumpolar, and includes southern and sub-Antarctic Australia, most birds within Australia’s jurisdiction come from New Zealand populations (BirdLife International 2004, Rexer-Huber 2017).
Population estimates and trends
While there is no global population trend information available for the species over three generations due to a lack of data, the population is considered to be declining (BirdLife International 2018j). There were an estimated 1,118,000 breeding pairs in 2019 (ACAP 2022).
Habitat critical to survival of species
The species is limited to 73 breeding sites on island groups of France (Crozet Islands, Kerguelen Islands), New Zealand (Antipodes Islands, Auckland Islands, Campbell Islands), South Africa (Prince Edward Islands), and other (Falkland Islands/Islas Malvinas, South Georgia/Islas Georgias del Sur), with the largest population at South Georgia/Islas Georgias del Sur (ACAP 2012y).
Threats
The risk matrix for the White-chinned Petrel is provided at Table 38, with the threats occurring in Australia’s jurisdiction highlighted.
Table 38: White-chinned Petrel (Procellaria aequinoctialis) risk matrix.
Likelihood of occurrence | Consequences |
Not significant | Minor | Moderate | Major | Catastrophic |
Almost certain | | Climate variability and change: variation in Southern Oscillation Index Marine pollution: marine plastics ingestion | Fisheries interactions: pelagic longline, demersal longline, trawl, artisanal Introduced pest species: predation by cats, black rats, Norwegian rats | | |
Likely | | | | | |
Possible | | | | | |
Unlikely | | | | | |
Rare or Unknown | | Human disturbance: take for food | | | |
Note: Threats occurring in Australia’s jurisdiction are highlighted in bold.
Family: Diomedeidae
Taxonomy
Procellaria parkinsoni Gray 1862 is accepted nomenclature for the Black Petrel. Originally Procellaria parkinsoni Gray 1862. The Black Petrel has previously been considered polytypic with Procellaria westlandica. (Westland Petrel) until Jackson (1958) proposed recognition at the specific level based on demographic differences, a view also supported by morphological and genetic differences (Marchant & Higgins 1990, Nunn & Stanley 1998). ACAP has concluded on advice from its Taxonomy Working Group that available data warrant recognition of the Black Petrel at the specific level with the nomenclature widely accepted (Brooke et al. 2008, ACAP 2012e).
Current status of taxon
Environment Protection and Biodiversity Conservation Act 1999 (Commonwealth): not listed
Biodiversity Conservation Act 2016 (Western Australia): not listed
National Parks and Wildlife Act 1972 (South Australia): not listed
Flora and Fauna Guarantee Act 1988 (Victoria): not listed
Threatened Species Protection Act 1995 (Tasmania): not listed
Biodiversity Conservation Act 2016 (New South Wales): not listed
Nature Conservation Act 1992 (Queensland): Least Concern
IUCN Red list of Threatened Species: Vulnerable
Action Plan for Australian Birds 2020: population visiting Australia Vulnerable
Species description
A small Procellaria petrel, the Black Petrel is approximately 46 cm in length, 0.6-0.9 kg in weight, with a wing length of 33-36 cm, and bill length of 39-43 mm (ACAP 2015, Menkhorst et al. 2017). Tubenosed; separate nostrils on separate on a yellow to white plated bill with dark tip. Combination of black and silver plumage that becomes browner with age, all black except for undersides of primaries (Onley & Scofield 2007, BirdLife International 2018l).
Life history
Breeding locality | Jurisdiction |
Great Barrier Island, Little Barrier Island | New Zealand |
Endemic to New Zealand with two breeding sites on Great Barrier Island, and Little Barrier Island (ACAP 2012e). The Black Petrel is an annual breeder when successful (Imber 1987). Adults arrive at colonies from October with females laying a single egg from mid-November to late January that hatches after incubation period of around 8 weeks from early January to late March, with fledging of chicks occurring after 3-4 months in April/June (Imber et al. 2003, Bell et al 2011). Mean annual breeding success varies by location from 45-76% (ACAP 2012e). The Black Petrel predominantly disperses north easterly to the eastern tropical Pacific Ocean as far north as California. Juveniles return to colonies when 4-6 years of age and commence breeding when birds are 5-7 years of age (Imber 1987). Generation length is estimated at 14.2 years (Bird et al. 2020).
Species distribution in Australia
Australia is within the foraging range of the Black Petrel. An indicative distribution map is not available for this non-threatened species. Tracking studies and at-sea records indicate that dispersal from the breeding colonies is predominantly to the eastern tropical Pacific Ocean between California and Ecuador (Imber 1987, BirdLife International 2004, ACAP 2012e), with some birds recorded in waters adjacent to eastern Australia (Bell et al. 2011).
Population estimates and trends
The global population is considered as stable, however there is no population trend information available for the species over three generations (BirdLife International 2018l). There were an estimated 6950 breeding pairs in 2021 (ACAP 2022).
Habitat critical to survival of species
The species is limited to two breeding sites on Great Barrier Island, and Little Barrier Island (New Zealand), with the largest population on Great Barrier Island (ACAP 2012e).
Threats
The risk matrix for the Black Petrel is provided at Table 39, with the threats occurring in Australia's jurisdiction highlighted.
Table 39: Black Petrel (Procellaria parkinsoni) risk matrix.
Likelihood of occurrence | Consequences |
Not significant | Minor | Moderate | Major | Catastrophic |
Almost certain | | Climate variability and change: variation in Southern Oscillation Index Disease: avian pox virus | Fisheries interactions: pelagic longline, demersal longline Introduced pest species: predation by cats, black rats, Polynesian rats, habitat degradation by pigs | | |
Likely | | | | | |
Possible | | | | | |
Unlikely | | | | | |
Rare or Unknown | | | | | |
Note: Threats occurring in Australia’s jurisdiction are highlighted in bold.
Family: Procellariidae
Taxonomy
Procellaria westlandica Falla 1946 is accepted nomenclature for the Westland Petrel. Originally Procellaria parkinsoni westlandica Falla 1946. The Westland Petrel was originally considered polytypic with Procellaria parkinsoni (Black Petrel) until Jackson (1958) proposed recognition at the specific level based on demographic differences, a view also supported by morphological and genetic differences (Marchant & Higgins 1990, Nunn & Stanley 1998). ACAP has concluded on advice from its Taxonomy Working Group that available data warrant recognition of the Westland Petrel at the specific level with the nomenclature widely accepted (Brooke et al. 2008, ACAP 2012w).
Current status of taxon
Environment Protection and Biodiversity Conservation Act 1999 (Commonwealth): not listed
Biodiversity Conservation Act 2016 (Western Australia): not listed
National Parks and Wildlife Act 1972 (South Australia): not listed
Flora and Fauna Guarantee Act 1988 (Victoria): not listed
Threatened Species Protection Act 1995 (Tasmania): not listed
Biodiversity Conservation Act 2016 (New South Wales): not listed
Nature Conservation Act 1992 (Queensland): Least Concern
IUCN Red list of Threatened Species: Vulnerable
Action Plan for Australian Birds 2020: population visiting Australia Least Concern
Species description
A small Procellaria petrel, the Westland Petrel is approximately 50-55 cm in length, 0.9-1.4 kg in weight, with a wing length of 37-40 cm, and bill length of 46-53 mm (ACAP 2015, Menkhorst et al. 2017). Tubenosed; separate nostrils on a yellow plated bill with black tip. Combination of black and silver plumage that becomes browner with age, all black except for undersides of primaries (Onley & Scofield 2007, BirdLife International 2018m).
Life history
Breeding locality | Jurisdiction |
Punakaiki | New Zealand |
Endemic to New Zealand with one breeding site on the South Island at Punakaiki (ACAP 2012w). The Westland Petrel is an annual breeder when successful (Jackson 1958, Baker & Coleman 1977). Adults arrive at colonies from mid-February with females laying a single egg predominantly in May that hatches after incubation period of 8-9 weeks mostly in late July, with fledging of chicks occurring after 4-5 months in November-January ((Jackson 1958, Baker & Coleman 1977). Mean annual breeding success is 62% (ACAP 2012w). The Westland Petrel predominantly disperses eastwards across the Pacific Ocean to South America. Juveniles return to colonies when 3 years of age and commence breeding when birds are at least 5 years of age (Waugh et al. 2006). Generation length is estimated at 22.5 years (Bird et al. 2020).
Species distribution in Australia
Australia is within the foraging range of the Westland Petrel. An indicative distribution map is not available for this non-threatened species. Tracking studies and at-sea records indicate that dispersal from the breeding colonies is predominantly eastwards across the Pacific Ocean from 20-50°S to the coastline of Chile (BirdLife International 2004, Spear et al. 2005, ACAP 2012w), with some birds recorded in waters adjacent to south-east Australia (Baker et al. 2002).
Population estimates and trends
The global population trend is uncertain with no population trend information available for the species over three generations (BirdLife International 2018m). There were an estimated 6200 breeding pairs in 2019 (ACAP 2022).
Habitat critical to survival of species
The species is limited to one breeding site on the South Island of New Zealand at Punakaiki (ACAP 2012w).
Threats
The risk matrix for the Black Petrel is provided at Table 40, with the threats occurring in Australia's jurisdiction highlighted.
Table 40: Westland Petrel (Procellaria westlandica) risk matrix.
Likelihood of occurrence | Consequences |
Not significant | Minor | Moderate | Major | Catastrophic |
Almost certain | | Fisheries interactions: pelagic longline, demersal longline, artisanal Human disturbance: events due to artificial lighting, power line strikes, trampling of burrows Introduced pest species: predation by cats, rats, dogs, stoats, trampling of burrows and habitat degradation by cattle, goats | | | |
Likely | | | Climate variability and change: severe storms | | |
Possible | | | | | |
Unlikely | | | | | |
Rare or Unknown | | Human disturbance: take for food | | | |
Note: Threats occurring in Australia’s jurisdiction are highlighted in bold.































